0% found this document useful (0 votes)
85 views2 pagesThermodynamics Simulation Task
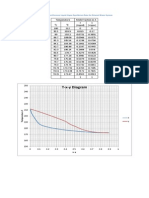
This document provides instructions for a coursework assignment on calculating vapor-liquid coexistence curves for a water-ethanol system using GROMACS. Students are asked to implement specific force fields for water and ethanol, find vapor pressure data from literature, prepare systems with different water-ethanol compositions, run simulations in the NVT ensemble at varying temperatures to calculate densities, plot results on an experimental phase diagram, and discuss if simulations match experiments and if water and ethanol can be separated by distillation.
Uploaded by
Malaak_wCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
85 views2 pagesThermodynamics Simulation Task
This document provides instructions for a coursework assignment on calculating vapor-liquid coexistence curves for a water-ethanol system using GROMACS. Students are asked to implement specific force fields for water and ethanol, find vapor pressure data from literature, prepare systems with different water-ethanol compositions, run simulations in the NVT ensemble at varying temperatures to calculate densities, plot results on an experimental phase diagram, and discuss if simulations match experiments and if water and ethanol can be separated by distillation.
Uploaded by
Malaak_wCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
/ 2