100% found this document useful (1 vote)
410 views11 pagesBoiling Heat Transfer Basics
1) Boiling heat transfer involves phase change between liquid and vapor and occurs at the solid-liquid interface when the surface temperature is above the saturation temperature.
2) There are two main modes of boiling - pool boiling where the liquid is stationary and convection boiling where external forces induce liquid motion.
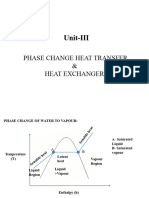
3) The boiling curve shows that extremely high heat fluxes are possible during nucleate boiling due to bubble nucleation, growth, and detachment from the surface, which effectively transports latent heat of vaporization away from the surface.
Uploaded by
Rahul KotadiyaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
410 views11 pagesBoiling Heat Transfer Basics
1) Boiling heat transfer involves phase change between liquid and vapor and occurs at the solid-liquid interface when the surface temperature is above the saturation temperature.
2) There are two main modes of boiling - pool boiling where the liquid is stationary and convection boiling where external forces induce liquid motion.
3) The boiling curve shows that extremely high heat fluxes are possible during nucleate boiling due to bubble nucleation, growth, and detachment from the surface, which effectively transports latent heat of vaporization away from the surface.
Uploaded by
Rahul KotadiyaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
/ 11