0% found this document useful (0 votes)
190 views24 pagesAdvanced Distillation Analysis
This document provides a multi-part problem to determine various parameters for separating a mixture of benzene and toluene using distillation. It involves calculating:
1) The minimum reflux ratio using McCabe-Thiele and Ponchon-Savarit methods for different feed conditions, including 25°C, boiling point, and 100°C.
2) The feed condition (subcooled liquid) and q-value using enthalpy calculations.
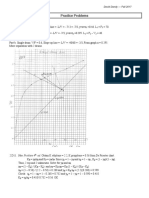
3) The number of equilibrium stages required using a Ponchon-Savarit graph with enthalpy-concentration data.
4) The reboiler and condenser duties using material balances and enthalpy values along the
Uploaded by
elha e. maruquinCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
190 views24 pagesAdvanced Distillation Analysis
This document provides a multi-part problem to determine various parameters for separating a mixture of benzene and toluene using distillation. It involves calculating:
1) The minimum reflux ratio using McCabe-Thiele and Ponchon-Savarit methods for different feed conditions, including 25°C, boiling point, and 100°C.
2) The feed condition (subcooled liquid) and q-value using enthalpy calculations.
3) The number of equilibrium stages required using a Ponchon-Savarit graph with enthalpy-concentration data.
4) The reboiler and condenser duties using material balances and enthalpy values along the
Uploaded by
elha e. maruquinCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
/ 24