0% found this document useful (0 votes)
381 views2 pages3-Entropy As A State Function
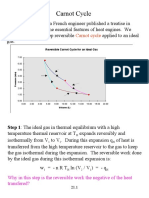
Entropy is a state function whose change for a cyclic process is zero. The Carnot cycle is a four stage reversible cycle consisting of two isothermal and two adiabatic processes. For the Carnot cycle, the total entropy change is zero since the heat absorbed from the high temperature reservoir equals the heat rejected to the low temperature reservoir. The efficiency of a heat engine depends only on the temperatures of the reservoirs and not on the details of the engine design according to Carnot's theorem.
Uploaded by
Ariel Raye RicaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
381 views2 pages3-Entropy As A State Function
Entropy is a state function whose change for a cyclic process is zero. The Carnot cycle is a four stage reversible cycle consisting of two isothermal and two adiabatic processes. For the Carnot cycle, the total entropy change is zero since the heat absorbed from the high temperature reservoir equals the heat rejected to the low temperature reservoir. The efficiency of a heat engine depends only on the temperatures of the reservoirs and not on the details of the engine design according to Carnot's theorem.
Uploaded by
Ariel Raye RicaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
/ 2