BC 2016 Additional problems on enzyme kinetics - KEY
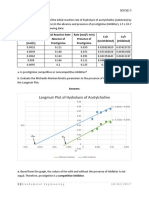
1. The following data were obtained from an enzyme kinetics experiment.
Graph the data using a Lineweaver-Burk plot and determine, by inspection of
the graph, the values for Km and Vmax.
[S] (µM) V (nmol/min)
_______ ___________
0.20 1.43
0.26 1.67
0.33 2.08
m
1.00 3.33
er as
co
eH w
o.
rs e
ou urc
o
aC s
vi y re
ed d
ar stu
Km=0.5 µM, Vmax= 5nmol/min
sh is
2. Calculate the maximum rate for an enzyme if its kcat = 1.4 x 104 s-1 Km = 90
µM. Is this enzyme very efficient?
Th
Km=90×10-6M
Specificity constant (or kinetic efficiency) = kcat/Km = 1.4×104/90×10-6 =
155.5×106s-1 M-1=1.55×108M-1s-1
Yes, it is efficient because the enzyme has achieved catalytic efficiency.
https://www.coursehero.com/file/18223974/BC2016-Additional-problems-enzyme-kinetics-7-key/
� 3. The effect of an inhibitor on an enzyme was tested and the experiment gave
the results below. Plot the data and determine, by inspection of the graph,
what type of inhibition is involved.
[S] µM V (µmol/min) V (µmol/min) V (µmol/min)
with 0.0 nM with 25 nM with 50 nM
Inhibitor Inhibitor Inhibitor
______ ___________ ___________ ___________
0.4 0.22 0.21 0.20
0.67 0.29 0.26 0.24
1.00 0.32 0.30 0.28
2.00 0.40 0.36 0.33
m
er as
co
eH w
o.
rs e
ou urc
o
aC s
vi y re
ed d
Uncompetitive
ar stu
4. You perform a kinetics experiment on the enzyme phosphatidylinositol
sh is
synthase (PI synthase) using as substrate radiolabeled inositol in a tracer
amount mixed with unlabeled inositol.
Th
PI Synthase
CDP-DAG + Inositol -----------------------------à Phosphatidylinositol
Using a scintillation counter, you have determined that the inositol substrate has a
specific radioactivity of 100 dpm (disintegrations per minute) / 1 nmol of inositol.
You collect the following data that represent the amount of radiolabeled
phosphatidylinositol formed (data in dpm) after a 10 min reaction using 5 µM enzyme
https://www.coursehero.com/file/18223974/BC2016-Additional-problems-enzyme-kinetics-7-key/
� [S] nmol/100 µl Reaction Volume dpm Recovered in Product Formed
____________________________ ____________________________
250 10,000
500 16,000
750 17,500
1,000 21,700
a) Determine nmol product formed per min for each substrate concentration used.
Velocity
[S] (mM)
(nmol/min)
2.5 10.0
5.0 16.0
m
er as
7.5 17.5
10.0 21.7
co
eH w
b) Prepare a Lineweaver-Burk plot of the data.
o.
rs e
ou urc
o
aC s
vi y re
ed d
ar stu
sh is
c) Determine Km and Vmax for PI synthase.
Th
Km=5.55mM, Vmax=33.3nmol/min
https://www.coursehero.com/file/18223974/BC2016-Additional-problems-enzyme-kinetics-7-key/
� 5. Salicylate (aspirin) inhibits the catalytic action of glutamate dehydrogenase.
Plot the data two ways: (1) v vs. [S] (2) 1/v vs. 1/[S] on graph paper. Estimate
the Vmax and Km in the presence and absence of this inhibitor.
Product per minute (microgram)
Substrate (mM) No Inhibitor 40mM Salicylate
1.5 0.21 0.08
2.0 0.25 0.1
3.0 0.28 0.12
4.0 0.33 0.13
8.0 0.39 0.15
16.0 0.46 0.17
Graph:
m
er as
co
eH w
o.
rs e
ou urc
o
aC s
vi y re
ed d
ar stu
sh is
Th
Vmax=0.5ug/min; Vmax'=0.2ug/min, Km=2.1mM
https://www.coursehero.com/file/18223974/BC2016-Additional-problems-enzyme-kinetics-7-key/
� a) How well do the estimates agree from the two plots.
Quite well. The [S] at half Vmax in the MMK graph is ~2mM which is
similar to that obtained thru the Line-Weaverburk plot
b) From the data, can you determine the type of inhibition?
Non-competitive
c) Determine the ratio of [ES] uninhibited to [ES] inhibited at 4mM [S].
Ratio of v/Vmax(uninhibited)/v/Vmax(inhibited) = 1.01
6. Another type of inhibitor of glutamate dehydrogenase was tested.
a) Do the same calculations on this inhibitor as in Q. 6.
b) From the data, can you determine the type of inhibition?
c) How well do they agree?
Product per minute (nanomol)
Substrate (mM) No Inhibitor 6mM Inhibitor
m
2.0 139 88
er as
3.0 179 121
co
4.0 213 149
eH w
10.0 313 257
o.
15.0 370 295
rs e
Graph: Must extrapolate by hand
ou urc
o
aC s
vi y re
ed d
ar stu
sh is
Th
Competitive; Vmax=500nmol/min; Km=5mM, Km'=10mM
Both graphs agree quite well. Vmax =500nmol/min. At 1/2Vmax (250nmol/min) the Km
for uninhibited is ~5.5mM, Km for inhibited is ~10mM.
https://www.coursehero.com/file/18223974/BC2016-Additional-problems-enzyme-kinetics-7-key/
Powered by TCPDF (www.tcpdf.org)