0% found this document useful (0 votes)
375 views40 pagesQuality Attributes in Pharma Design
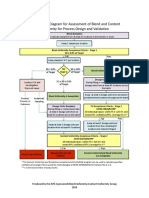
This document discusses approaches to identify critical quality attributes (CQAs), critical material attributes (CMAs), and critical process parameters (CPPs) under quality by design (QbD). It defines CQAs, CMAs, and CPPs and explains how they are related and how CMAs and CPPs can impact CQAs. The document provides an example approach to identify CQAs using a quality target product profile and an example approach to determine if a material attribute or process parameter is critical through experimentation and data analysis. It emphasizes that criticality is a continuum and the focus should be on controlling the vital few parameters that most impact CQAs.
Uploaded by
grandcrisCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
375 views40 pagesQuality Attributes in Pharma Design
This document discusses approaches to identify critical quality attributes (CQAs), critical material attributes (CMAs), and critical process parameters (CPPs) under quality by design (QbD). It defines CQAs, CMAs, and CPPs and explains how they are related and how CMAs and CPPs can impact CQAs. The document provides an example approach to identify CQAs using a quality target product profile and an example approach to determine if a material attribute or process parameter is critical through experimentation and data analysis. It emphasizes that criticality is a continuum and the focus should be on controlling the vital few parameters that most impact CQAs.
Uploaded by
grandcrisCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
/ 40