100% found this document useful (1 vote)
292 views2 pagesEnergy Level Diagrams - Worksheet
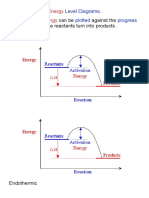
The document discusses energy level diagrams and how they relate to chemical reactions. It explains that energy level diagrams plot the change in energy against the progress of a reaction as reactants turn into products. The document also describes how reactions require an activation energy to break bonds in reactants and form bonds in products, and whether reactions release energy (exothermic) or absorb energy (endothermic) depending on the energy levels of reactants versus products.
Uploaded by
zarna nirmal rawalCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
292 views2 pagesEnergy Level Diagrams - Worksheet
The document discusses energy level diagrams and how they relate to chemical reactions. It explains that energy level diagrams plot the change in energy against the progress of a reaction as reactants turn into products. The document also describes how reactions require an activation energy to break bonds in reactants and form bonds in products, and whether reactions release energy (exothermic) or absorb energy (endothermic) depending on the energy levels of reactants versus products.
Uploaded by
zarna nirmal rawalCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
/ 2