0% found this document useful (0 votes)
40 views22 pagesSection 2a-1d Conduction Introduction
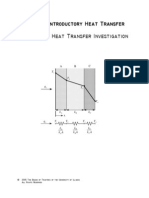
This document provides an introduction to 1D steady-state heat conduction. It defines key concepts like thermal conductivity, Fourier's law of heat conduction, and the heat diffusion equation. Thermal conductivity describes the rate of heat transfer and depends on the material. The heat diffusion equation relates the temperature distribution to thermal properties and any heat sources. Experiments are described to determine thermal conductivity and the importance of boundary conditions for solving the heat diffusion equation.
Uploaded by
Mustafa OCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
40 views22 pagesSection 2a-1d Conduction Introduction
This document provides an introduction to 1D steady-state heat conduction. It defines key concepts like thermal conductivity, Fourier's law of heat conduction, and the heat diffusion equation. Thermal conductivity describes the rate of heat transfer and depends on the material. The heat diffusion equation relates the temperature distribution to thermal properties and any heat sources. Experiments are described to determine thermal conductivity and the importance of boundary conditions for solving the heat diffusion equation.
Uploaded by
Mustafa OCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
/ 22