0% found this document useful (0 votes)
4 views4 pagesExperiment 3
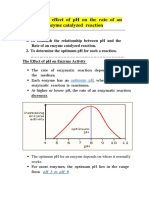
This document outlines an experiment to study the effect of pH on the activity of the enzyme acid phosphatase. It details the principles, expected outcomes, reagents, and protocols for determining the optimum pH for enzyme activity by measuring absorbance at 410 nm. The experiment emphasizes the importance of pH in enzyme function and provides precautions for safe handling of reagents.
Uploaded by
malihatanveer01Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
4 views4 pagesExperiment 3
This document outlines an experiment to study the effect of pH on the activity of the enzyme acid phosphatase. It details the principles, expected outcomes, reagents, and protocols for determining the optimum pH for enzyme activity by measuring absorbance at 410 nm. The experiment emphasizes the importance of pH in enzyme function and provides precautions for safe handling of reagents.
Uploaded by
malihatanveer01Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
/ 4