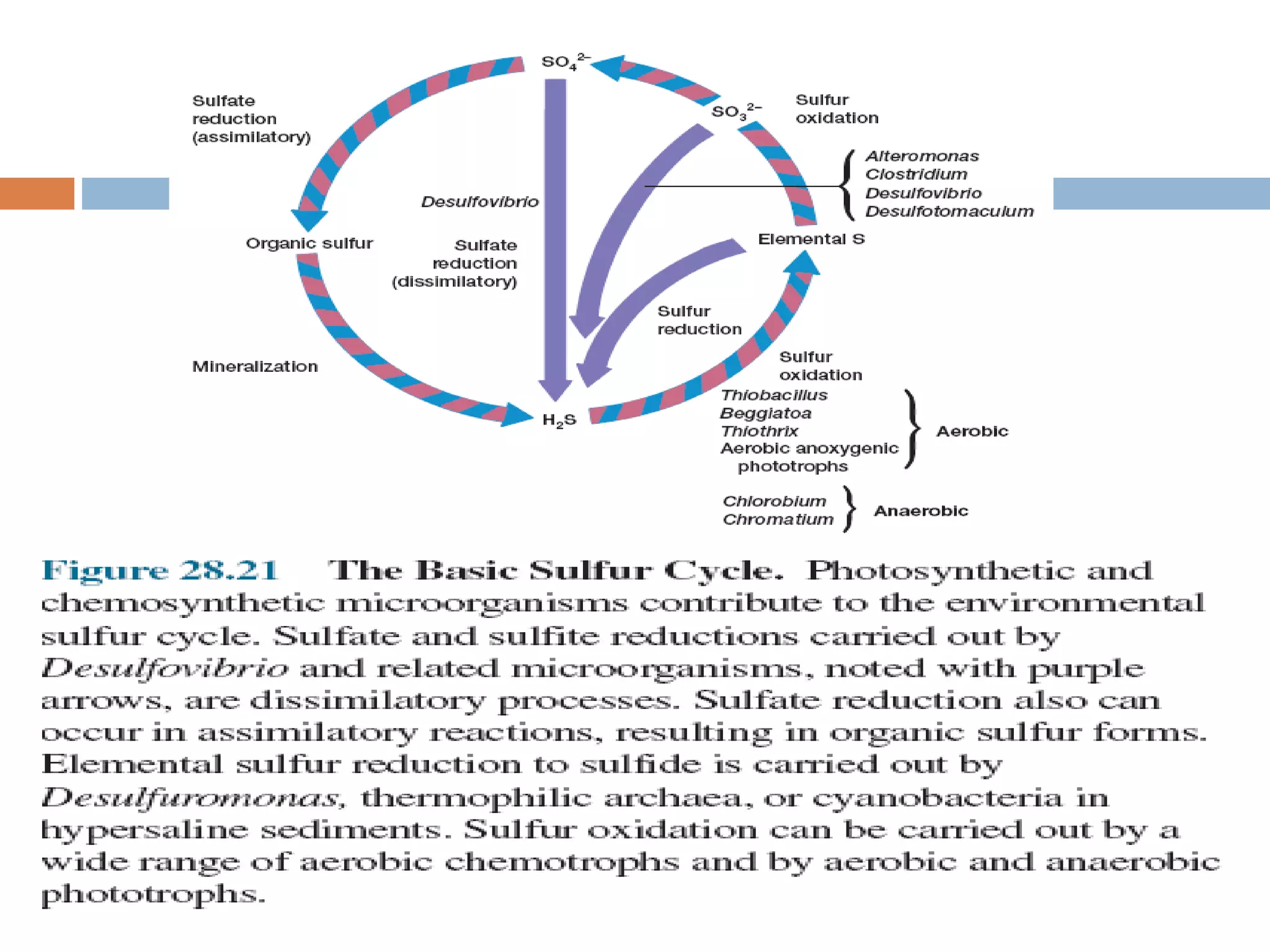
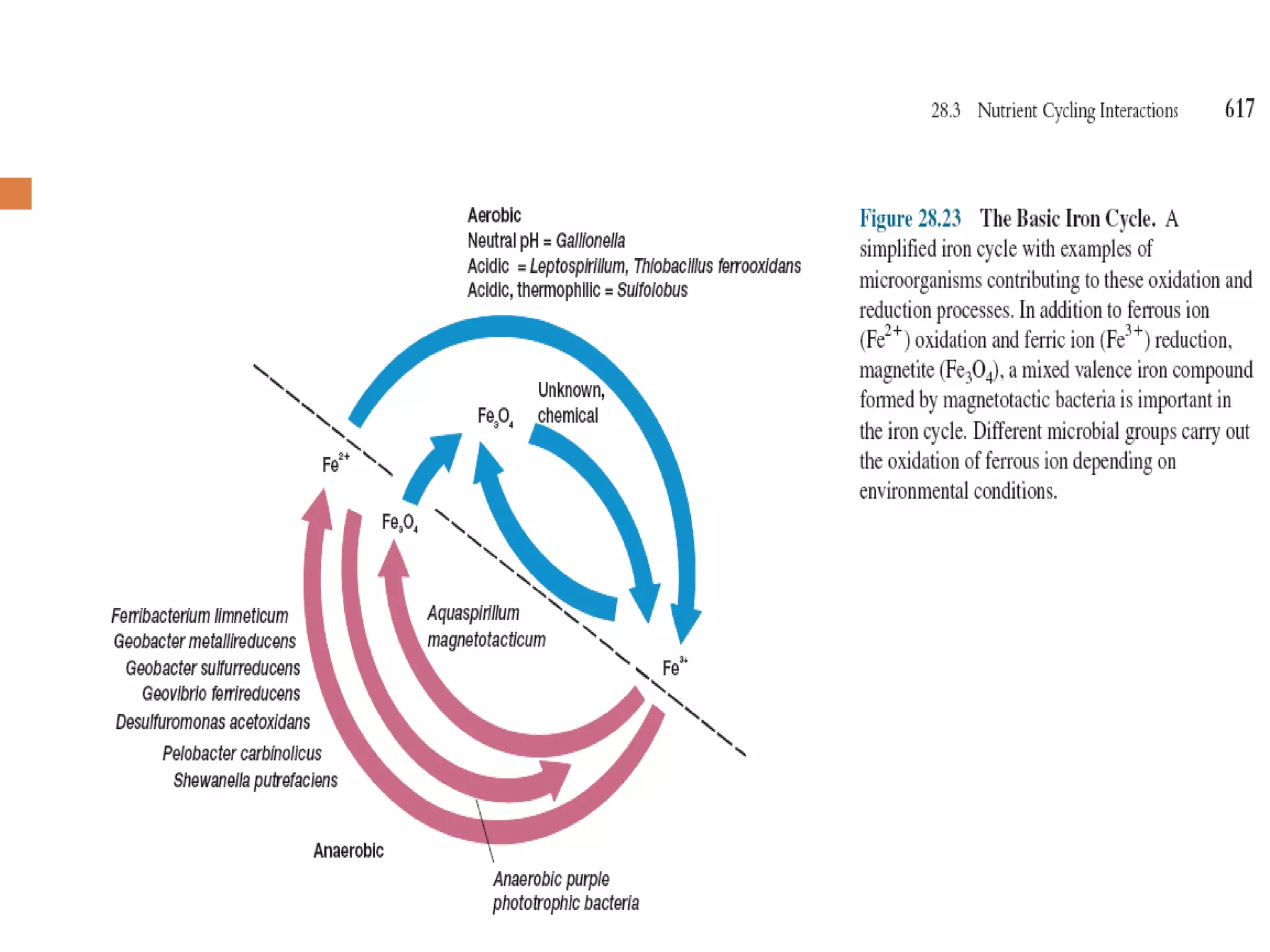
The document discusses the sulfur and iron cycles, highlighting the pivotal role microorganisms play in transforming and utilizing various sulfur and iron compounds for metabolic processes. It describes specific genera involved in sulfate and iron reduction, their ecological significances, and mentions chemical processes that occur abioticly in these cycles. Additionally, the text introduces the concept of magnetotactic bacteria and the recent discovery of microorganisms utilizing ferrous ions in anoxygenic photosynthesis.