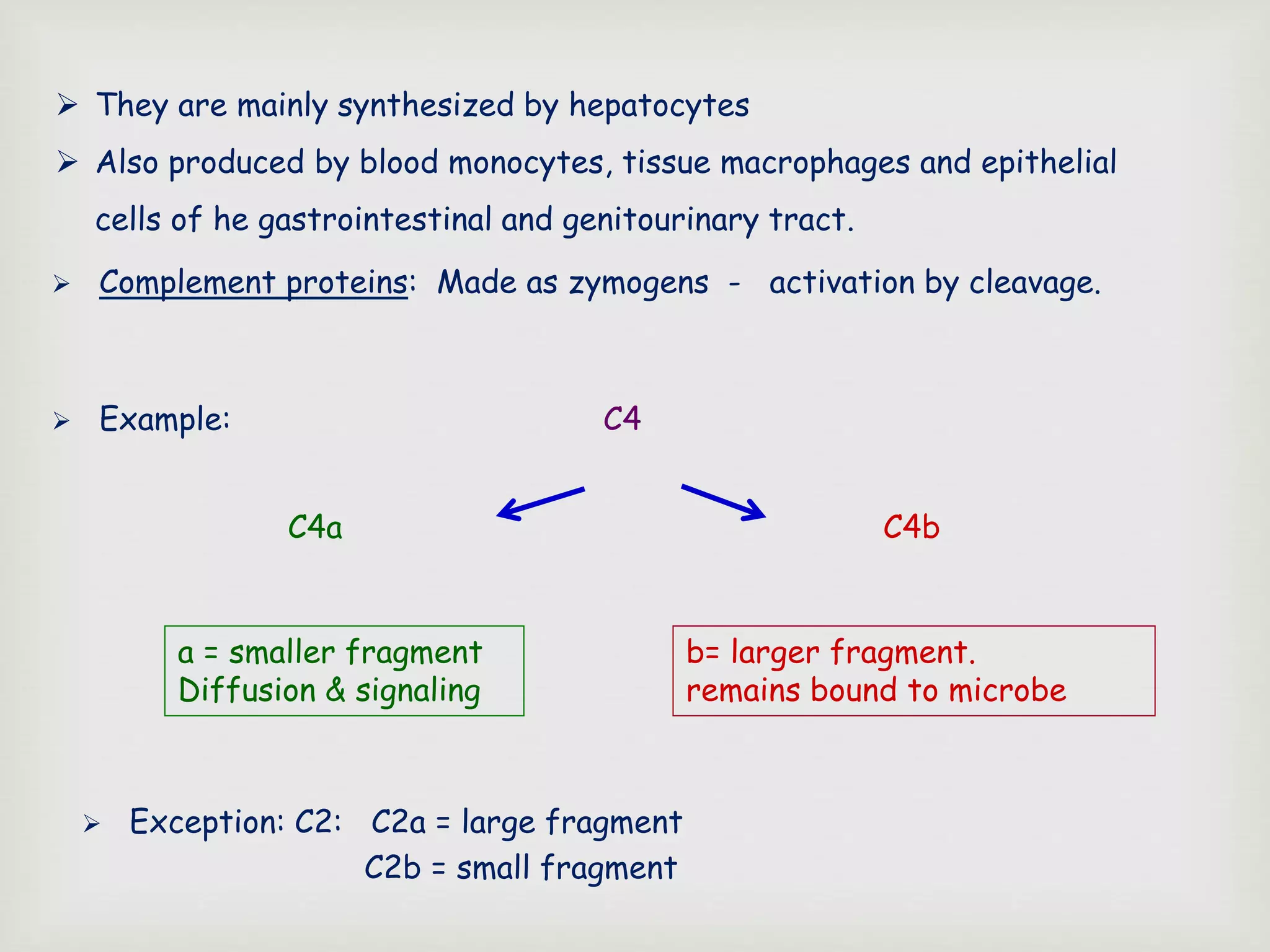
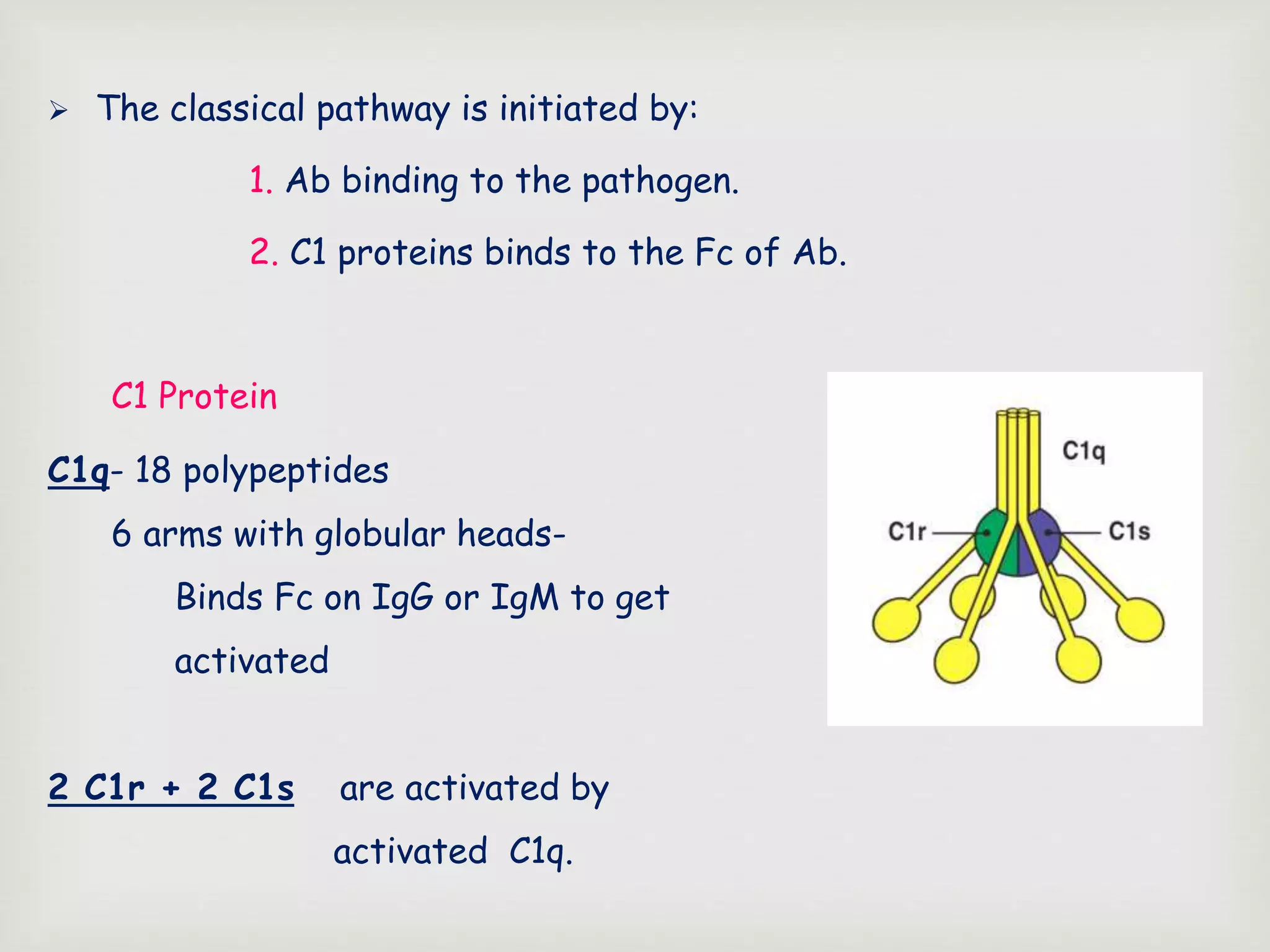
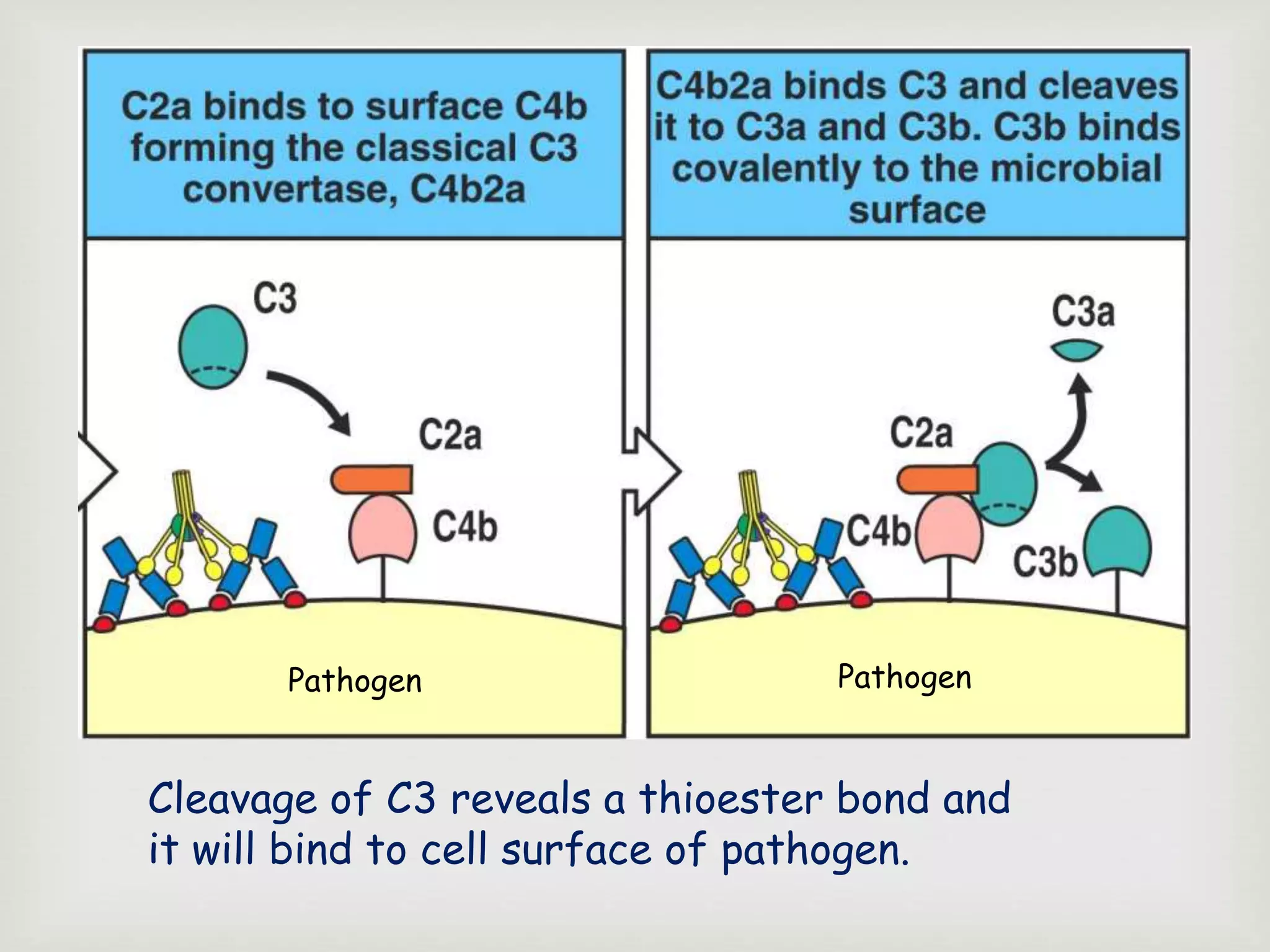
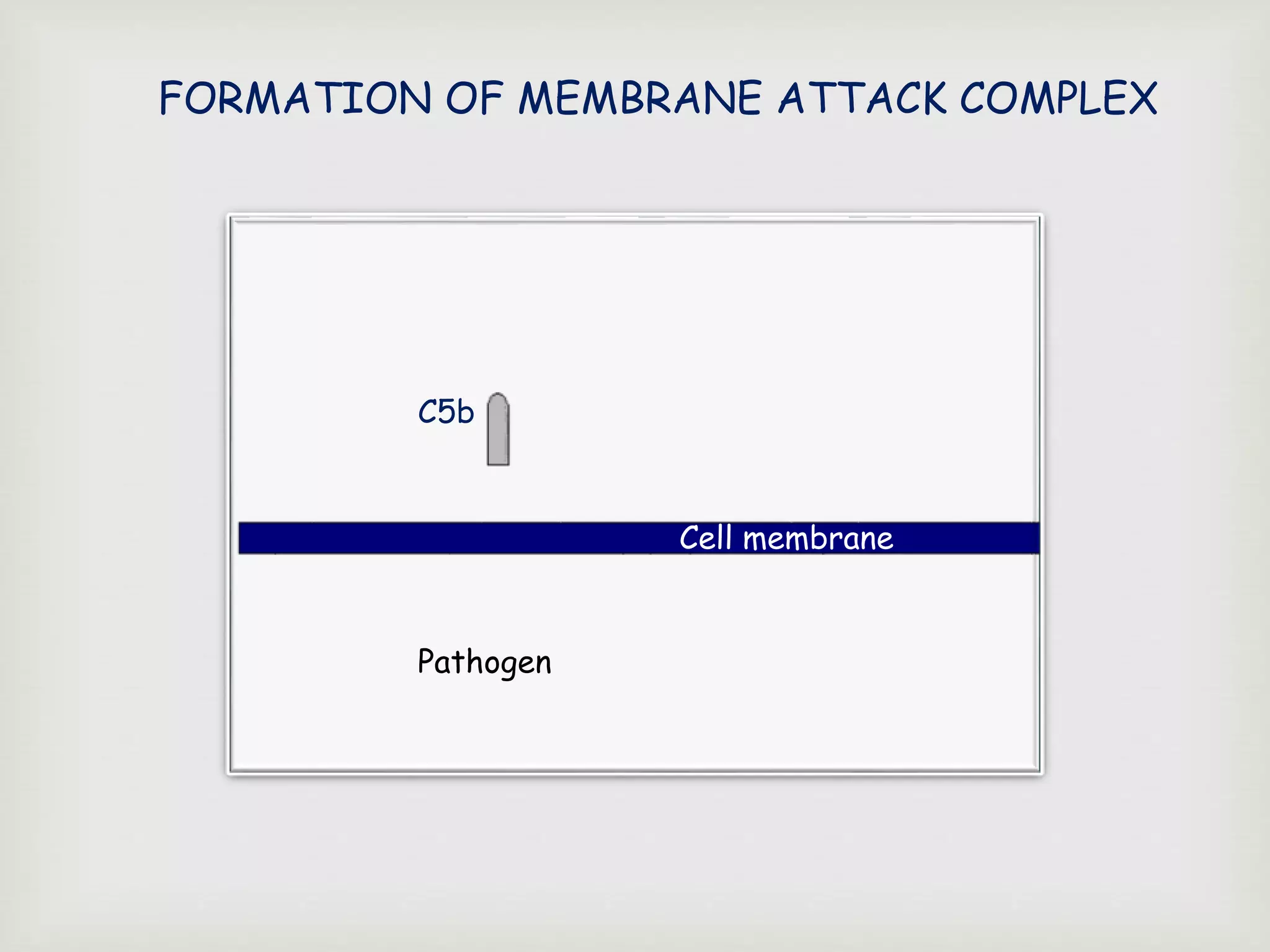
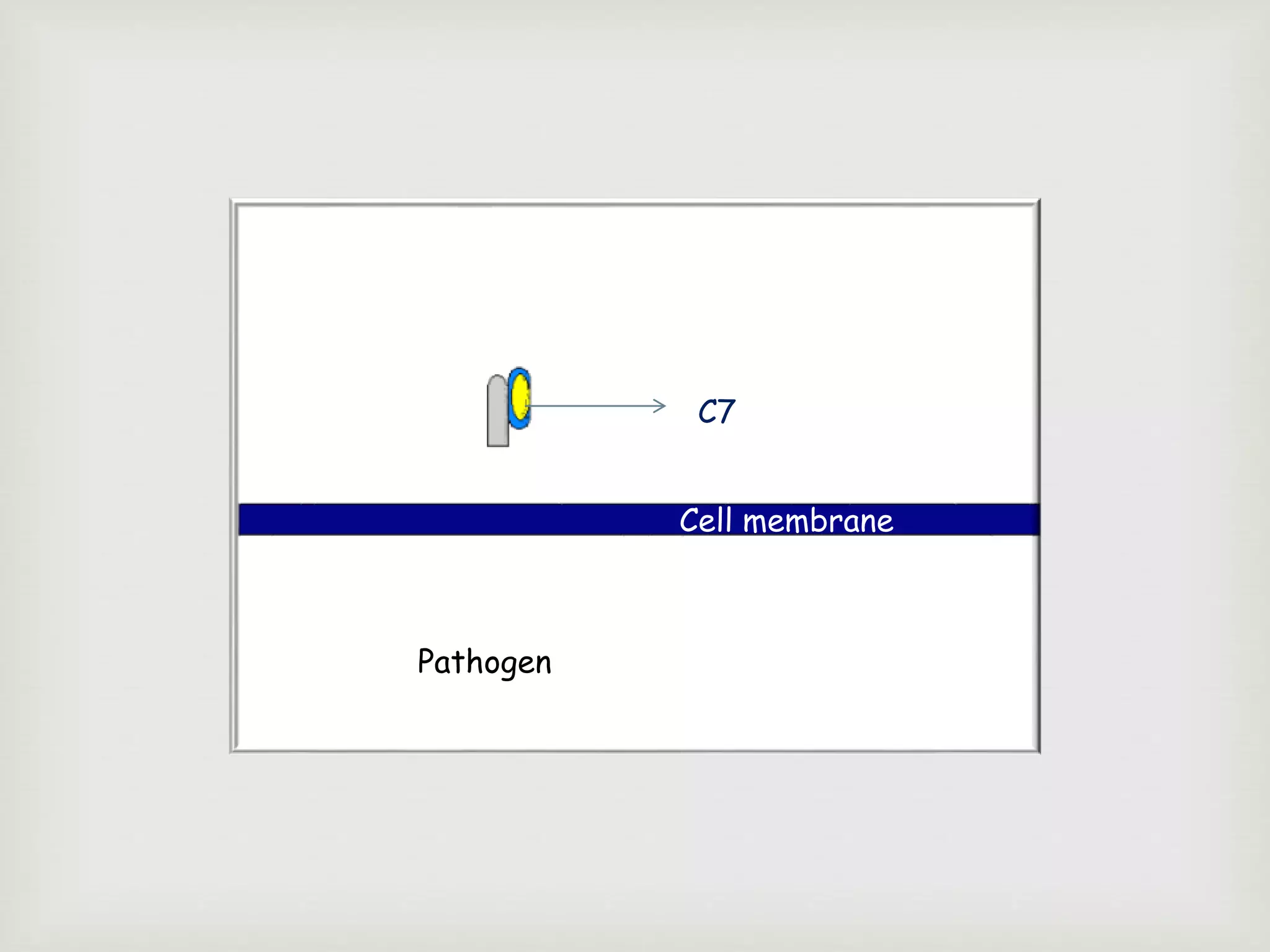
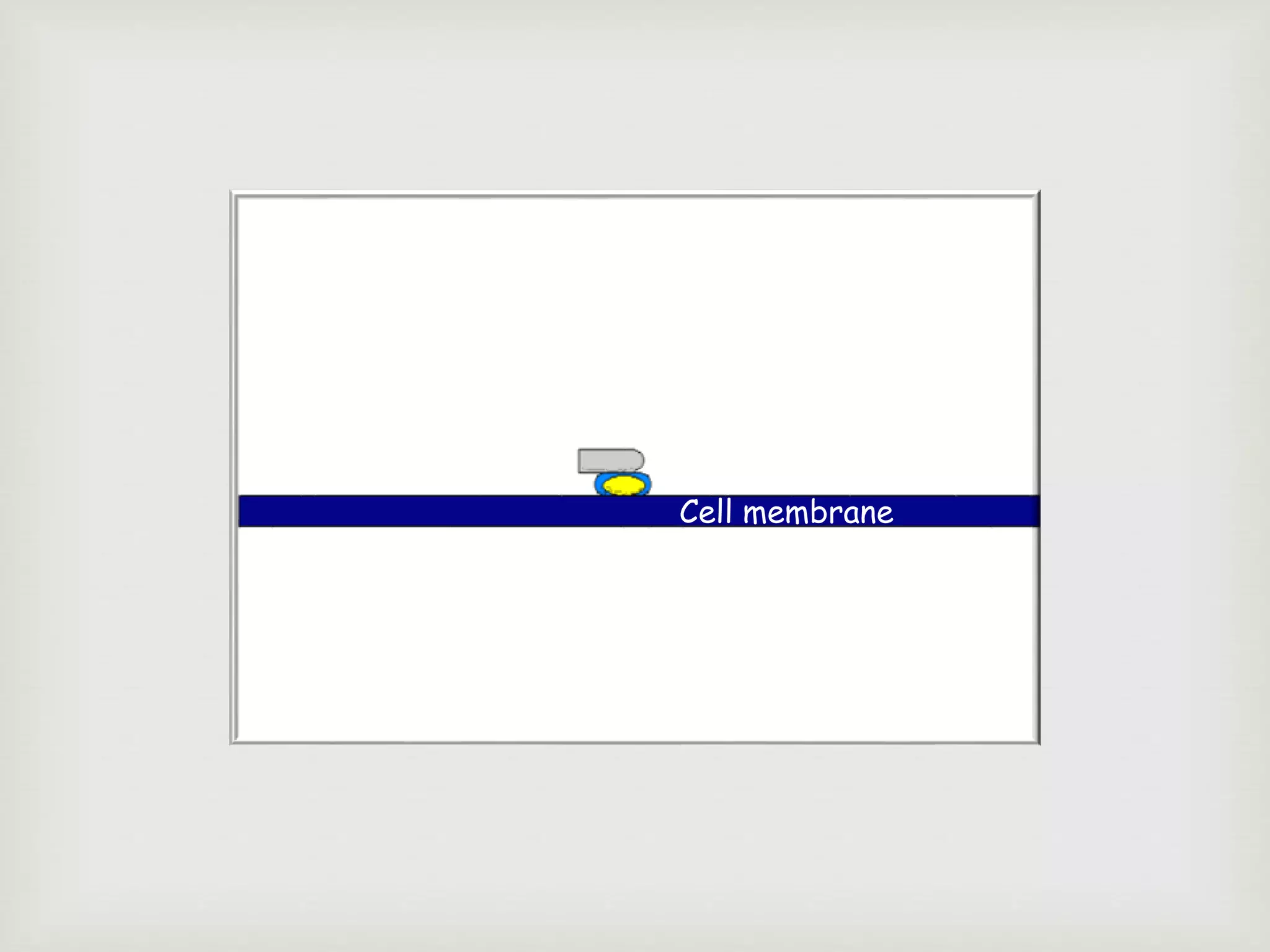
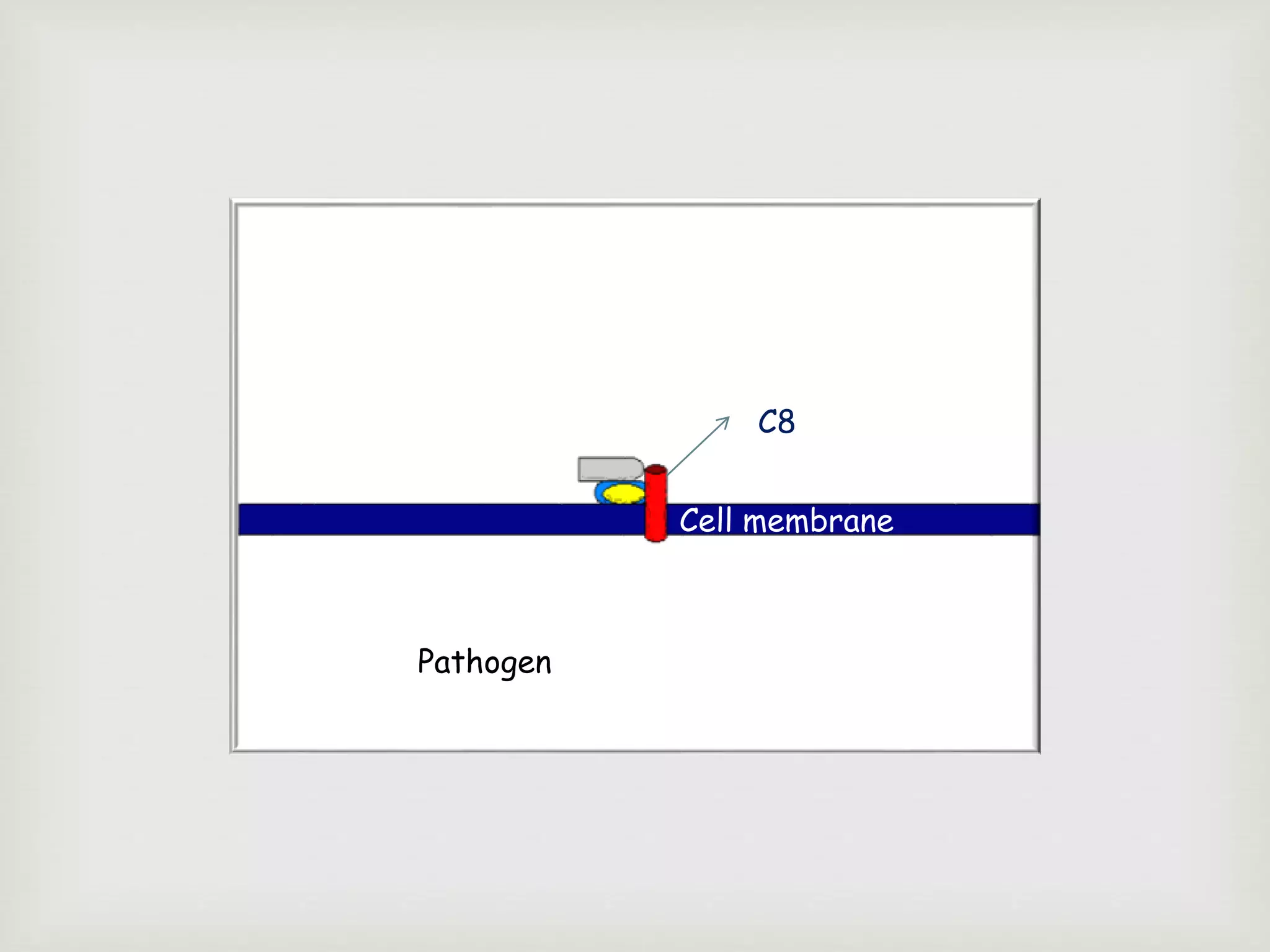
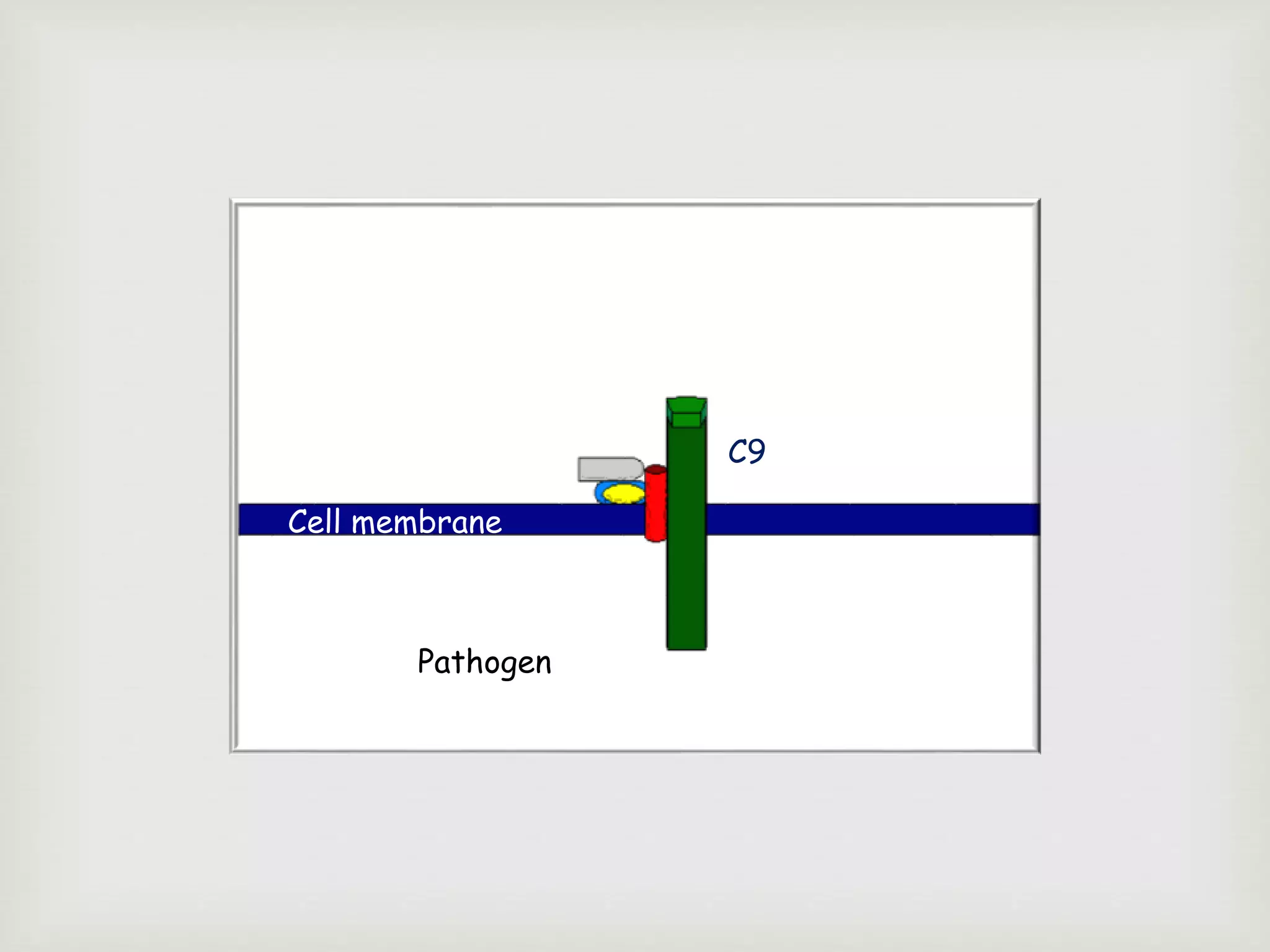
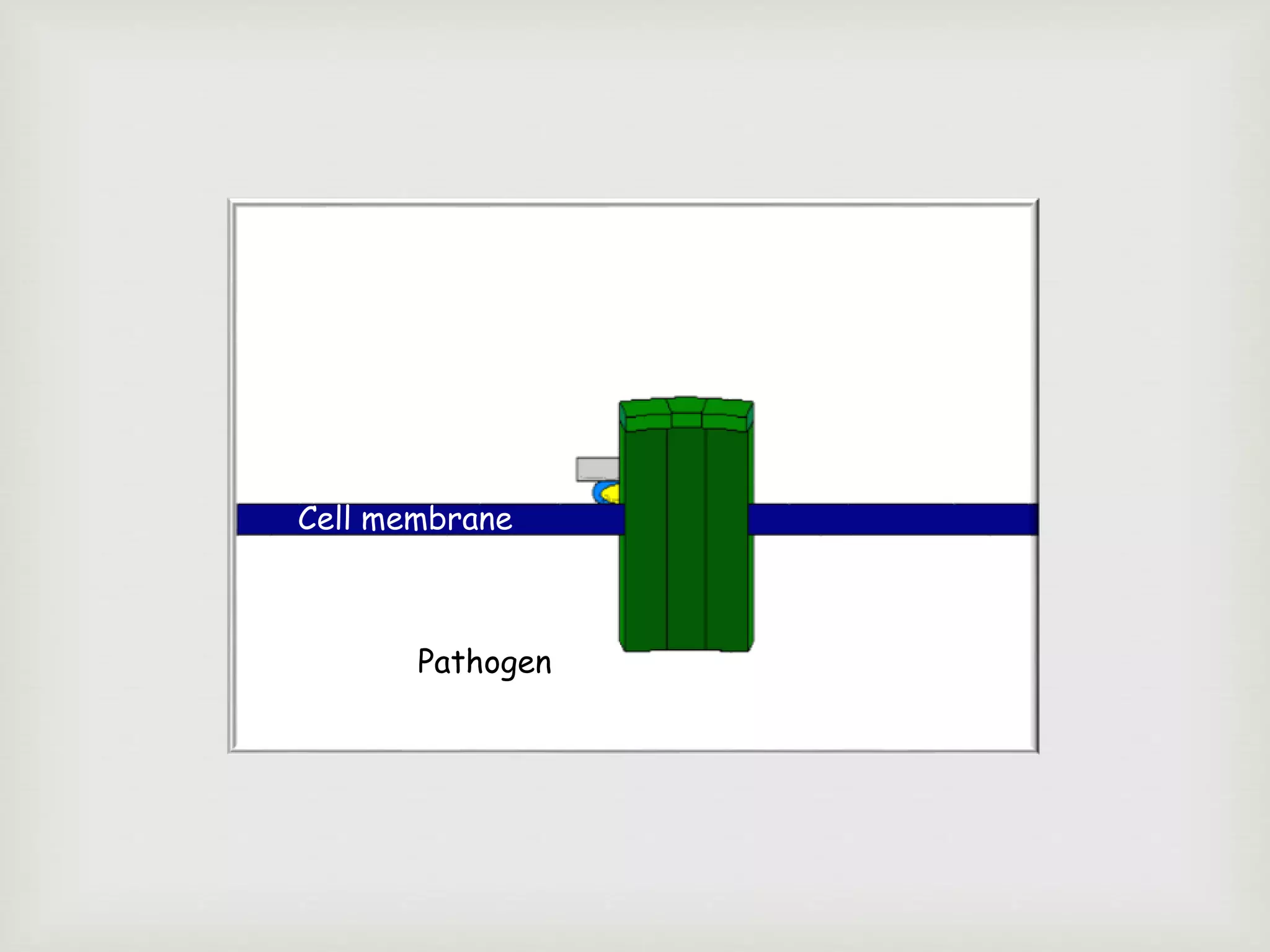
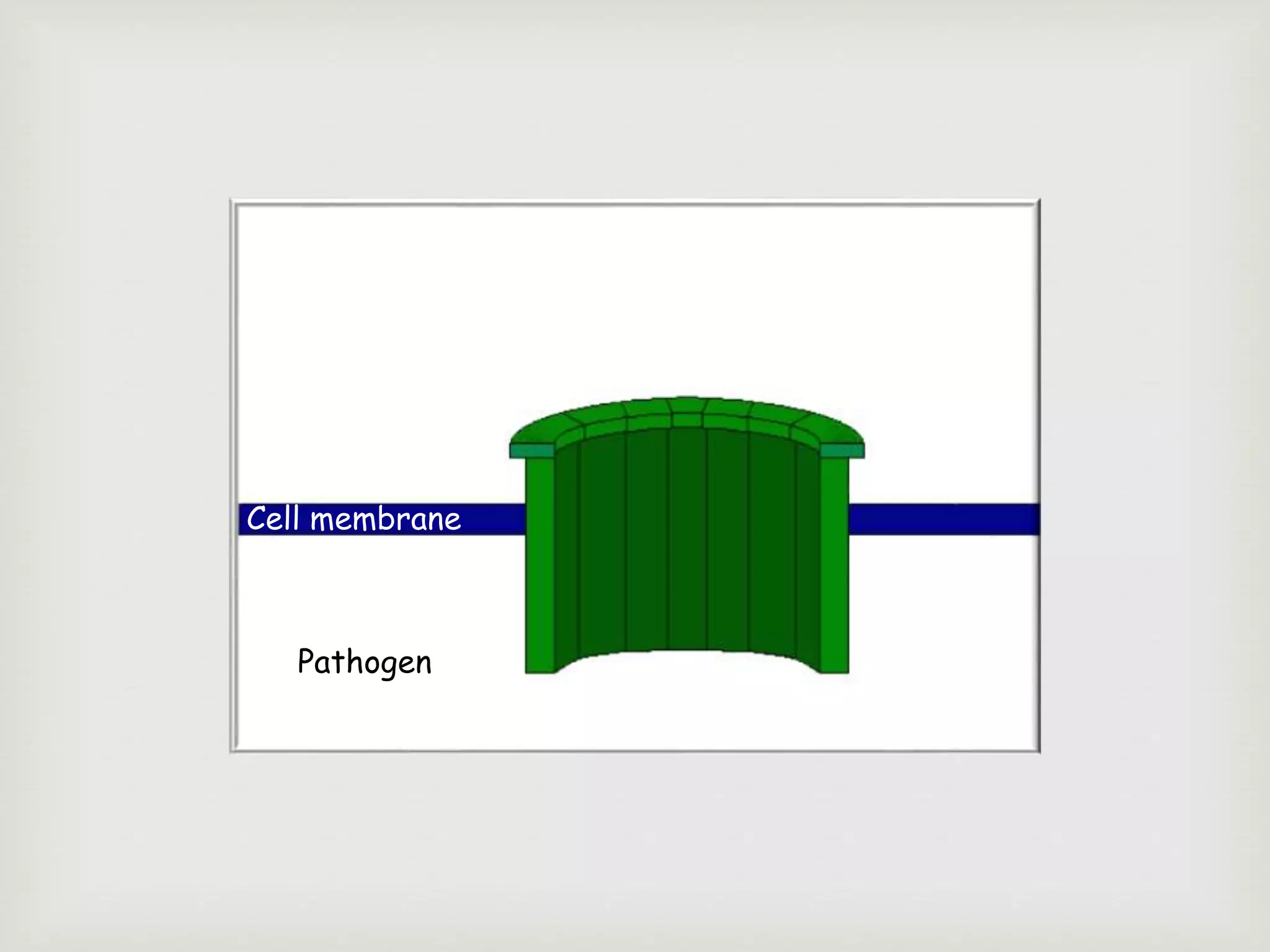
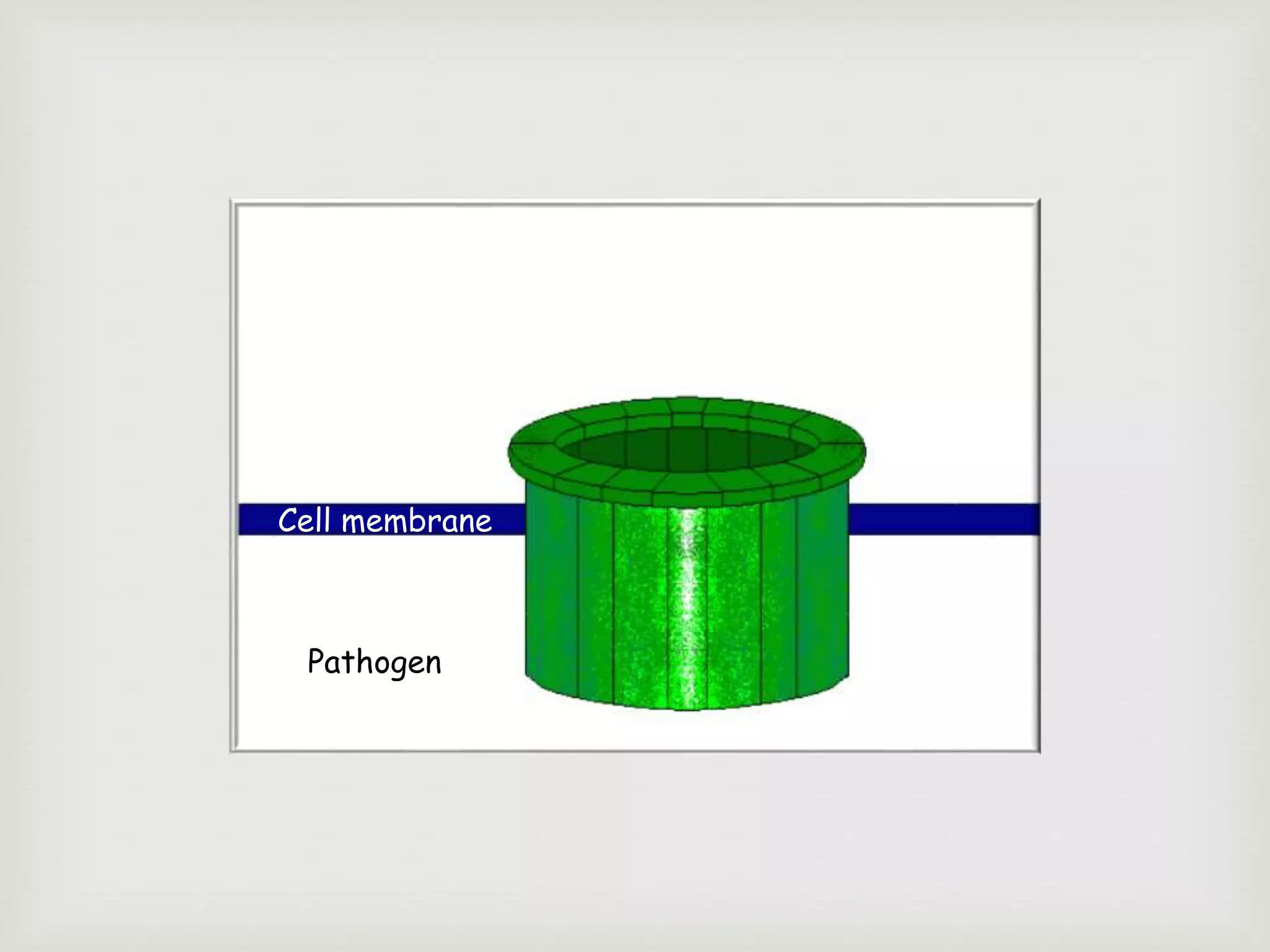
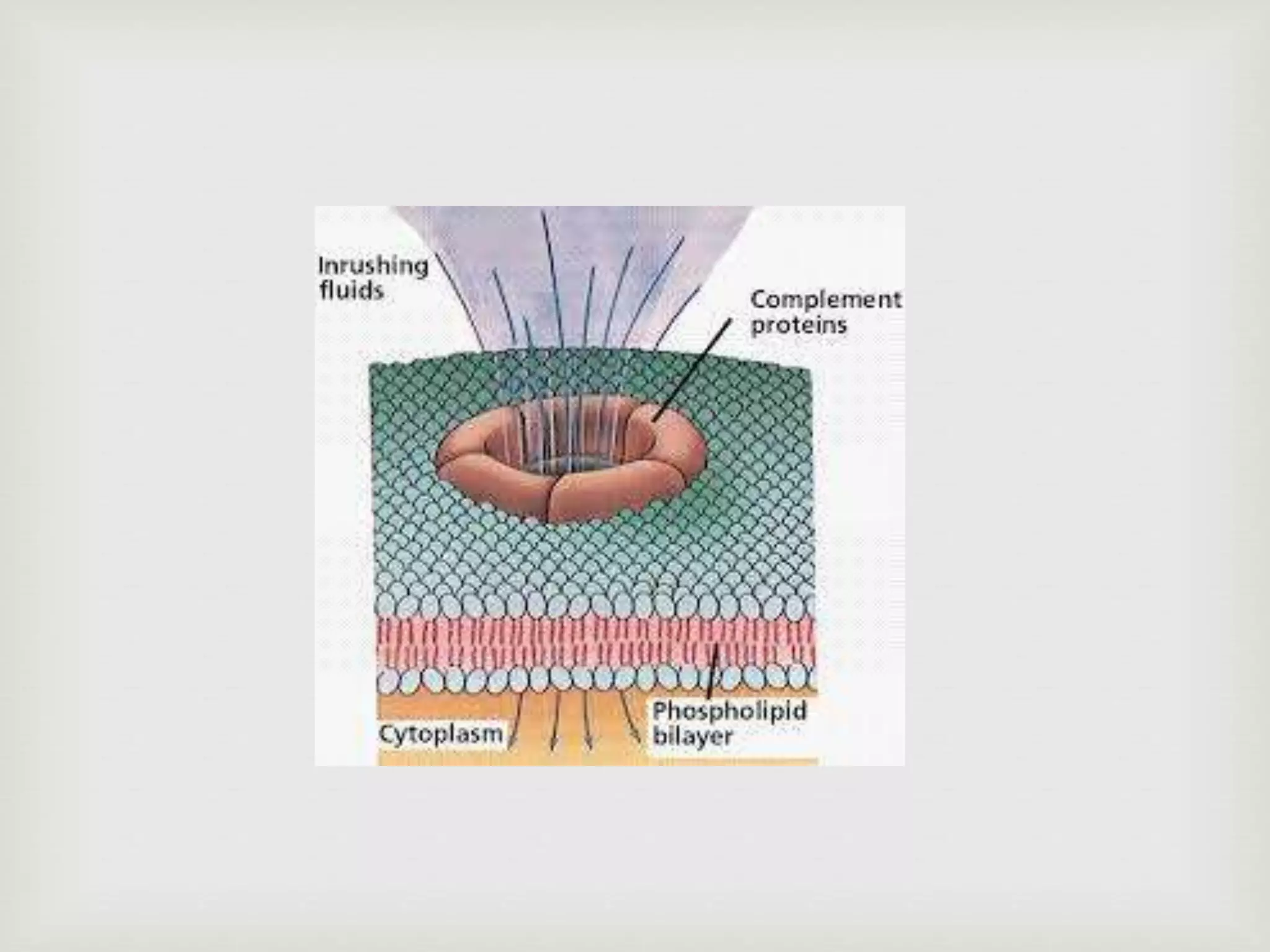
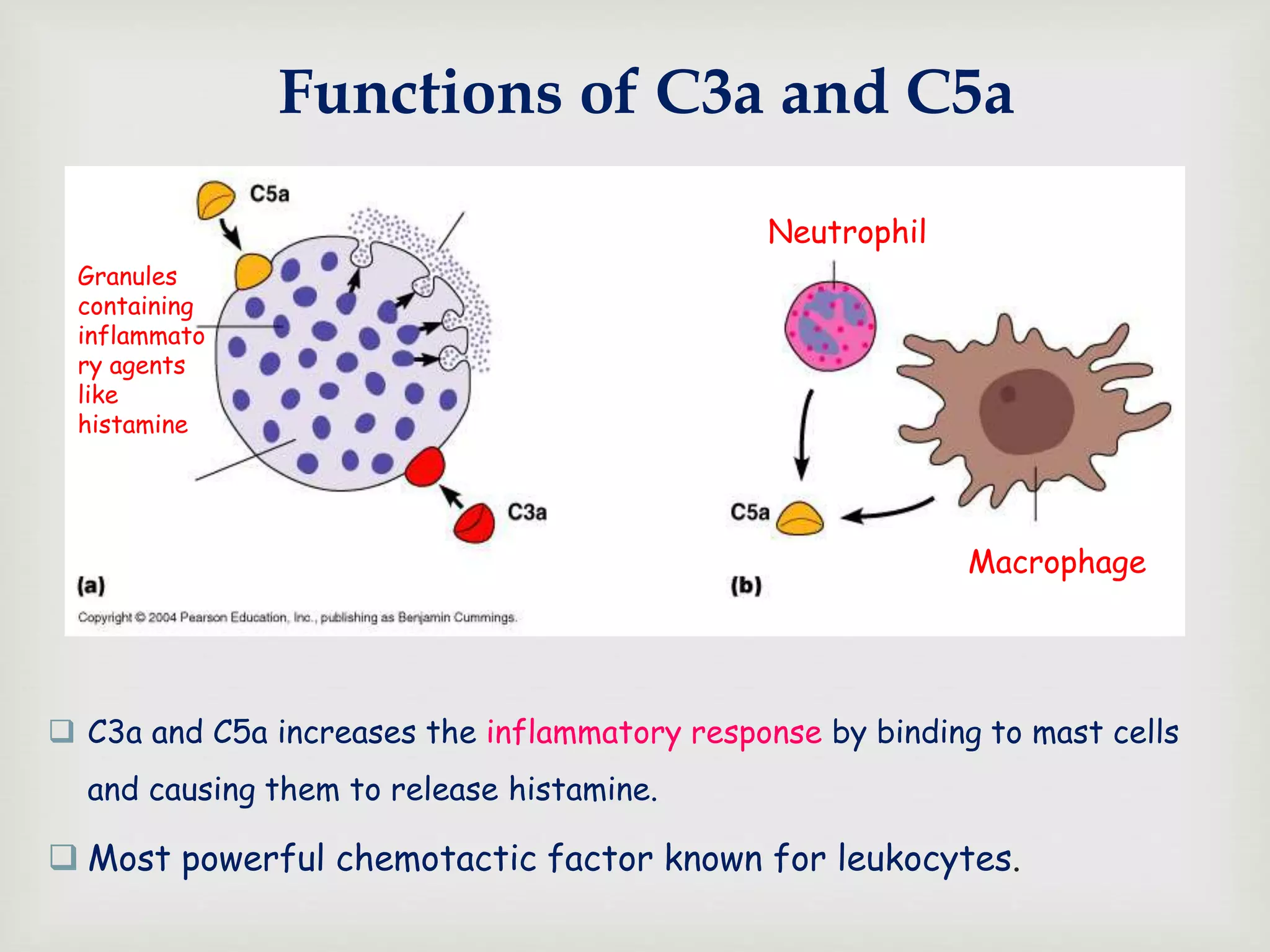
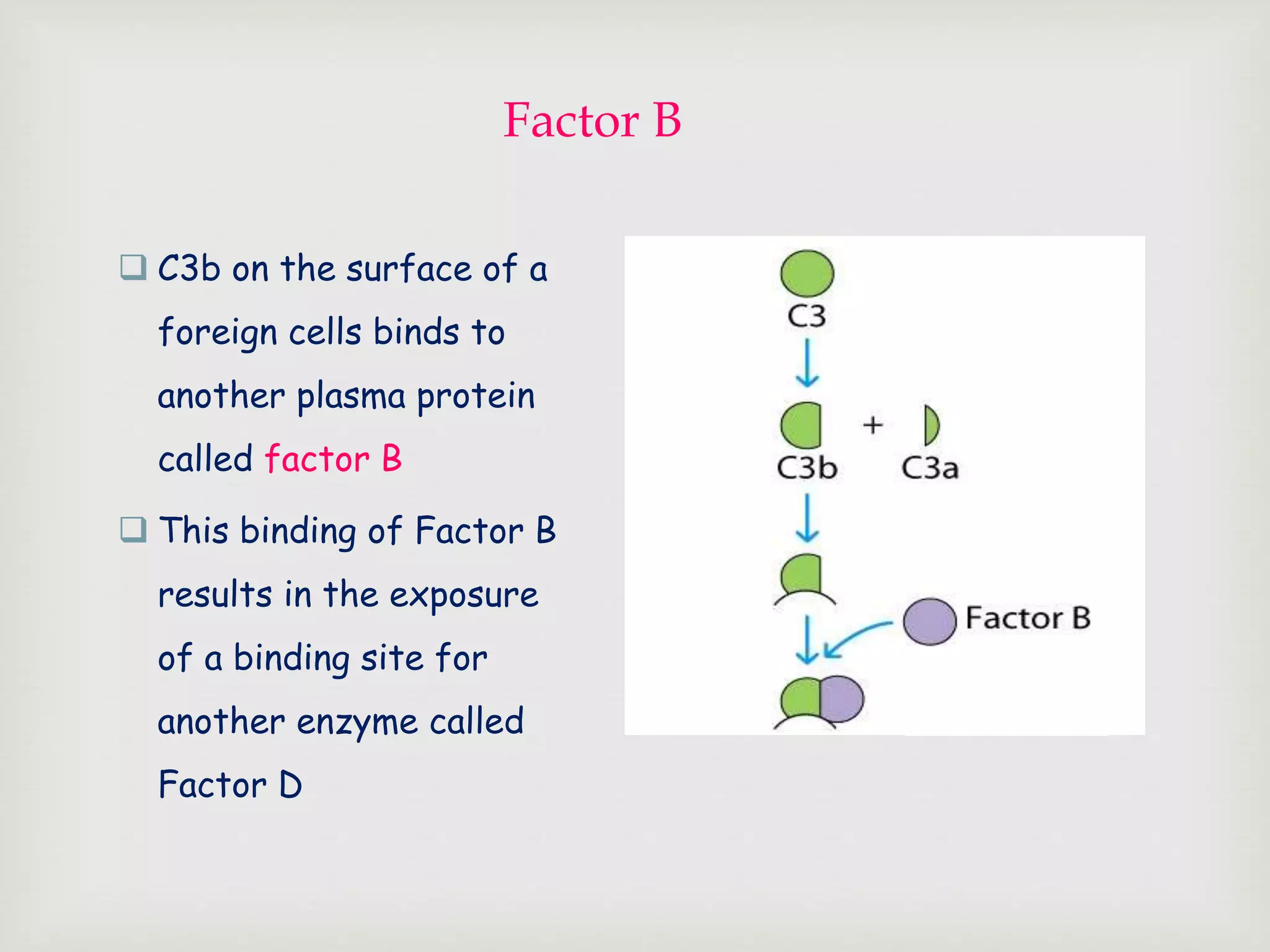
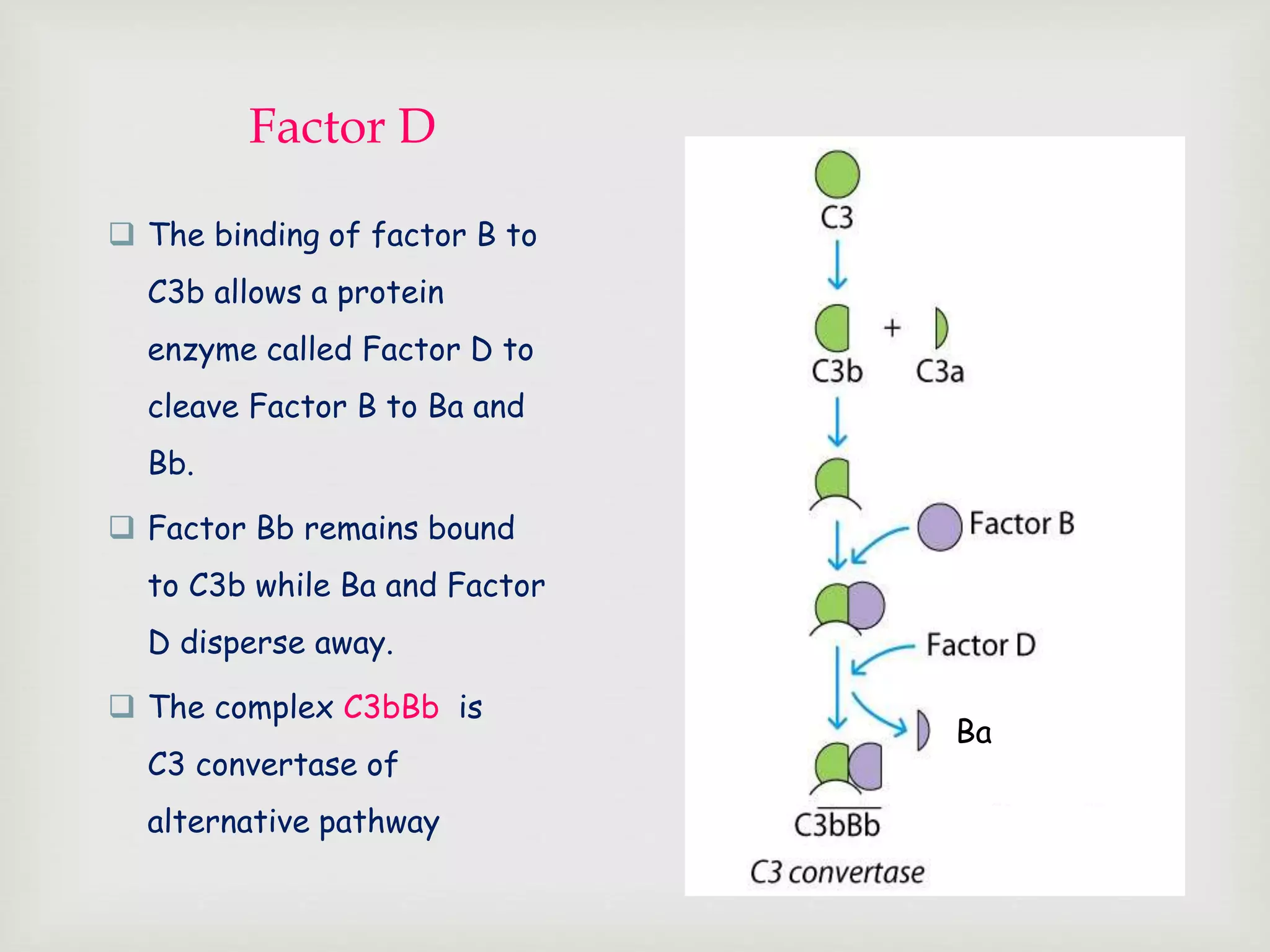
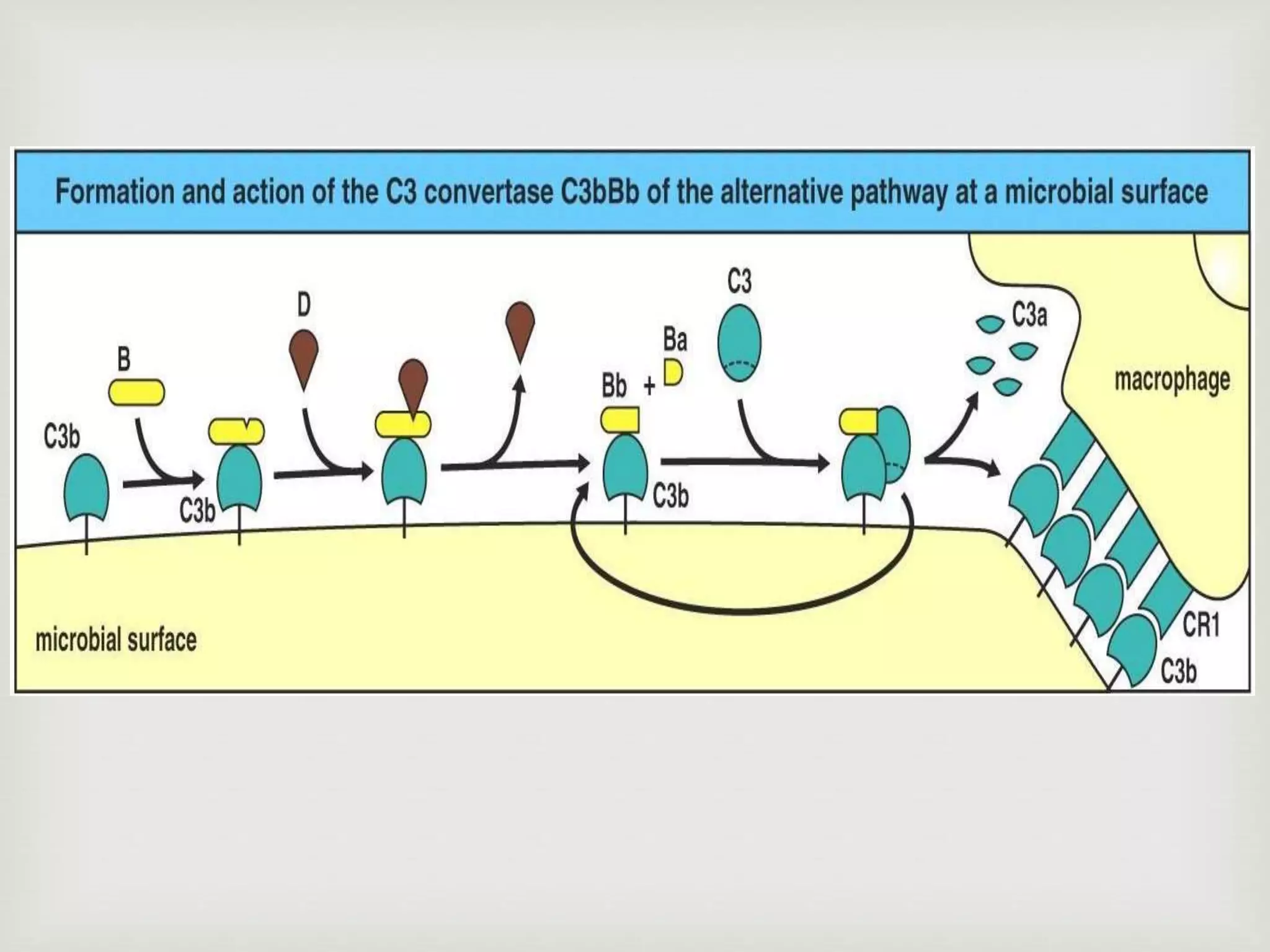
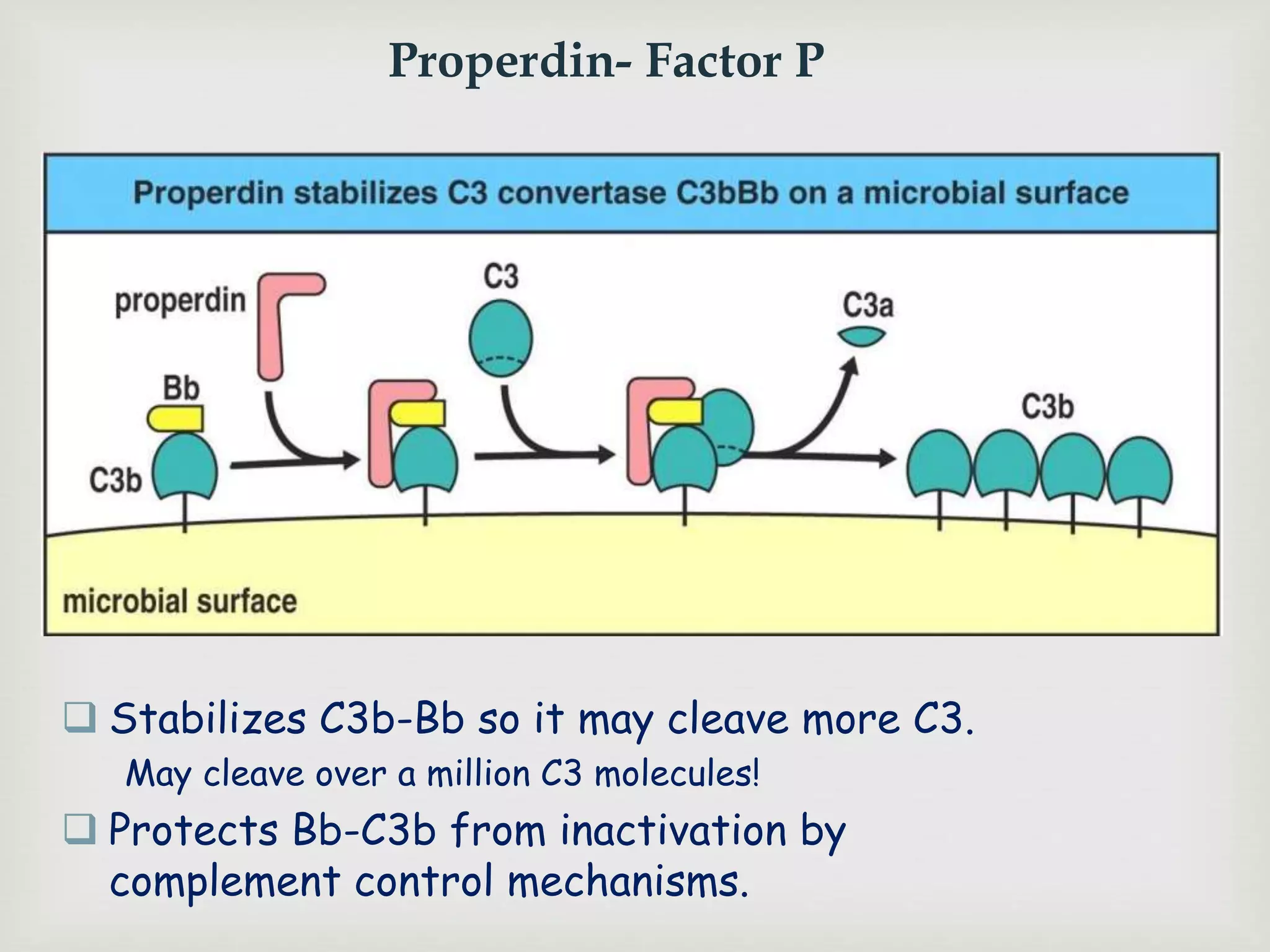
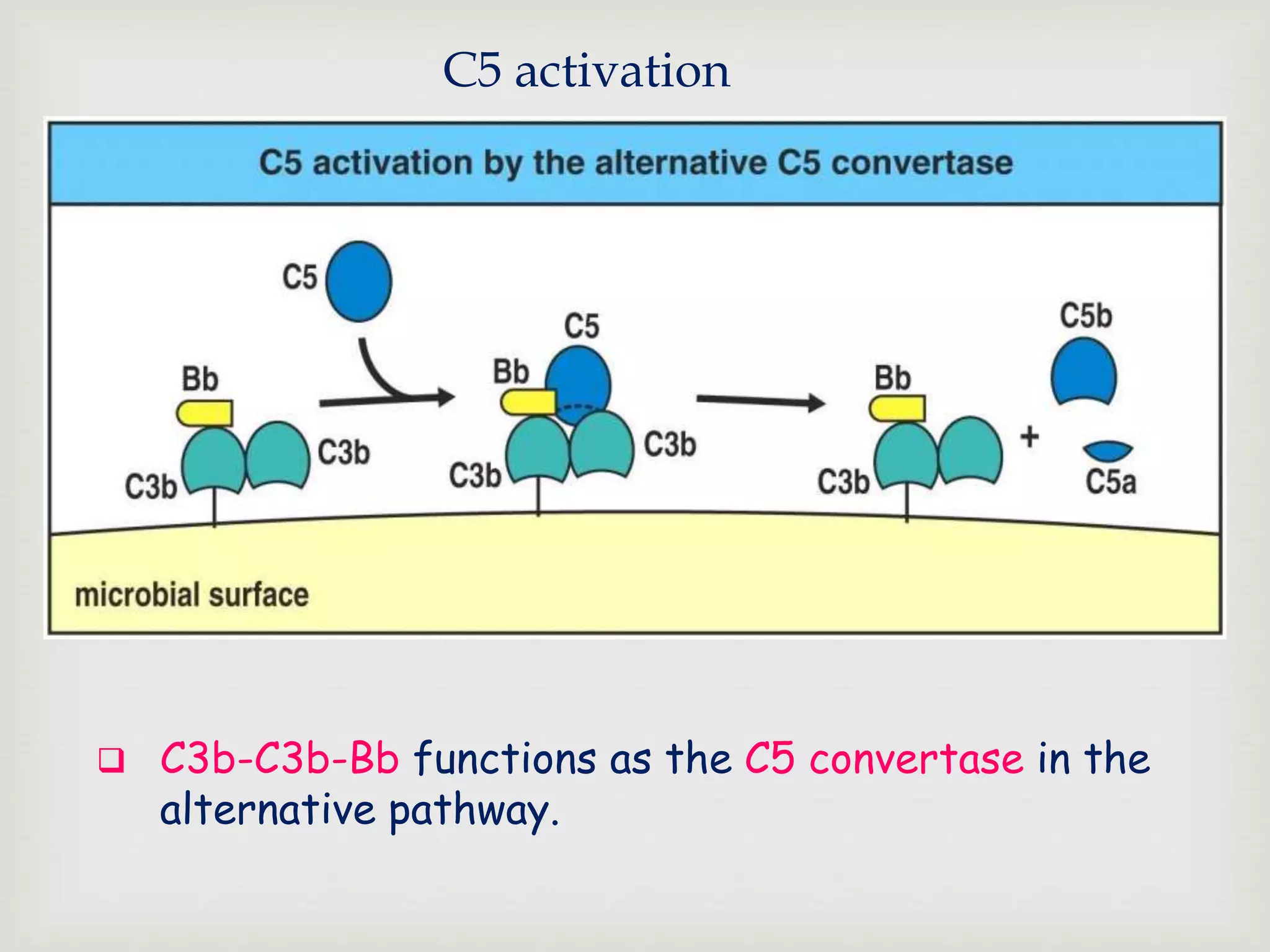
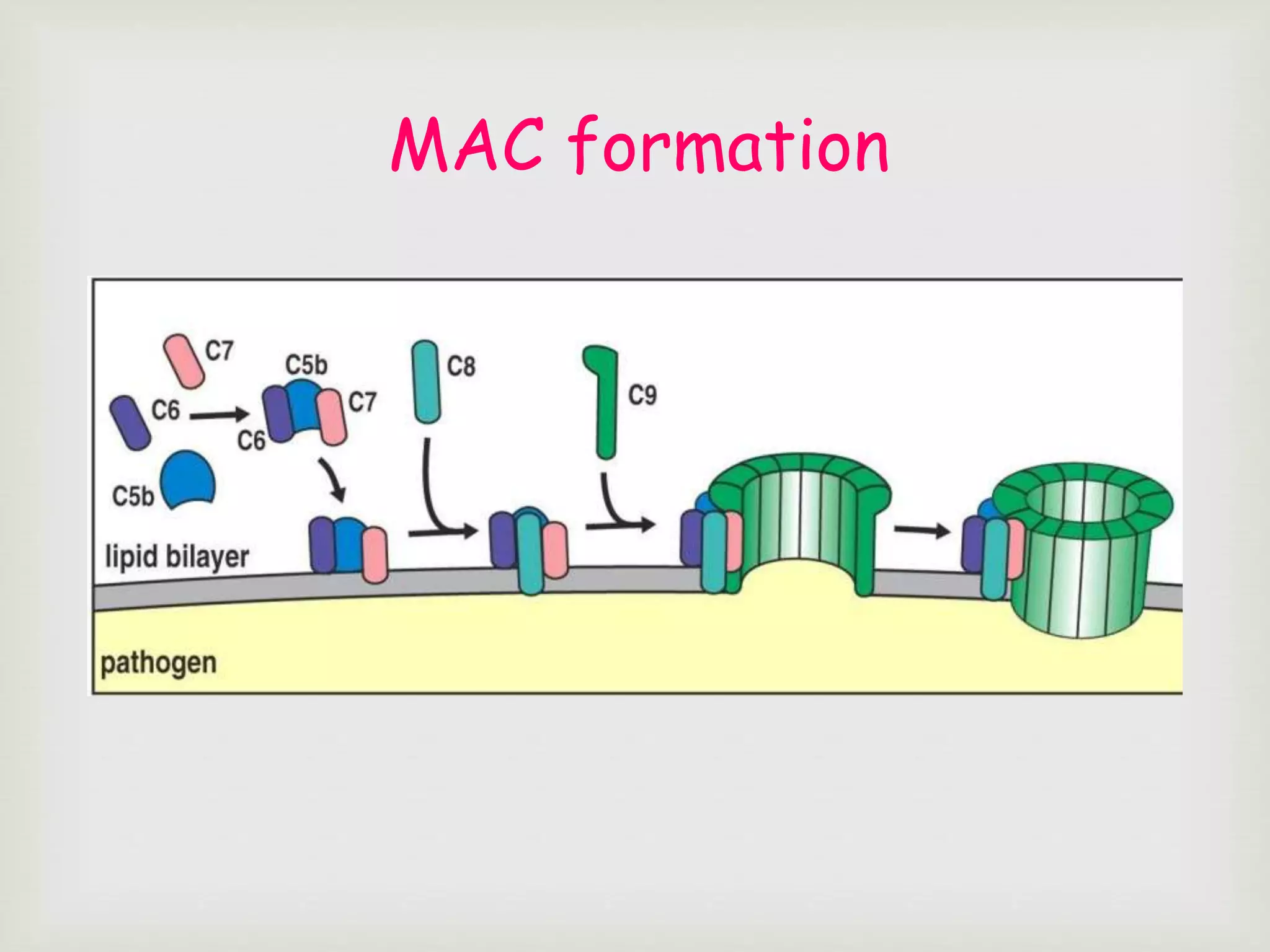
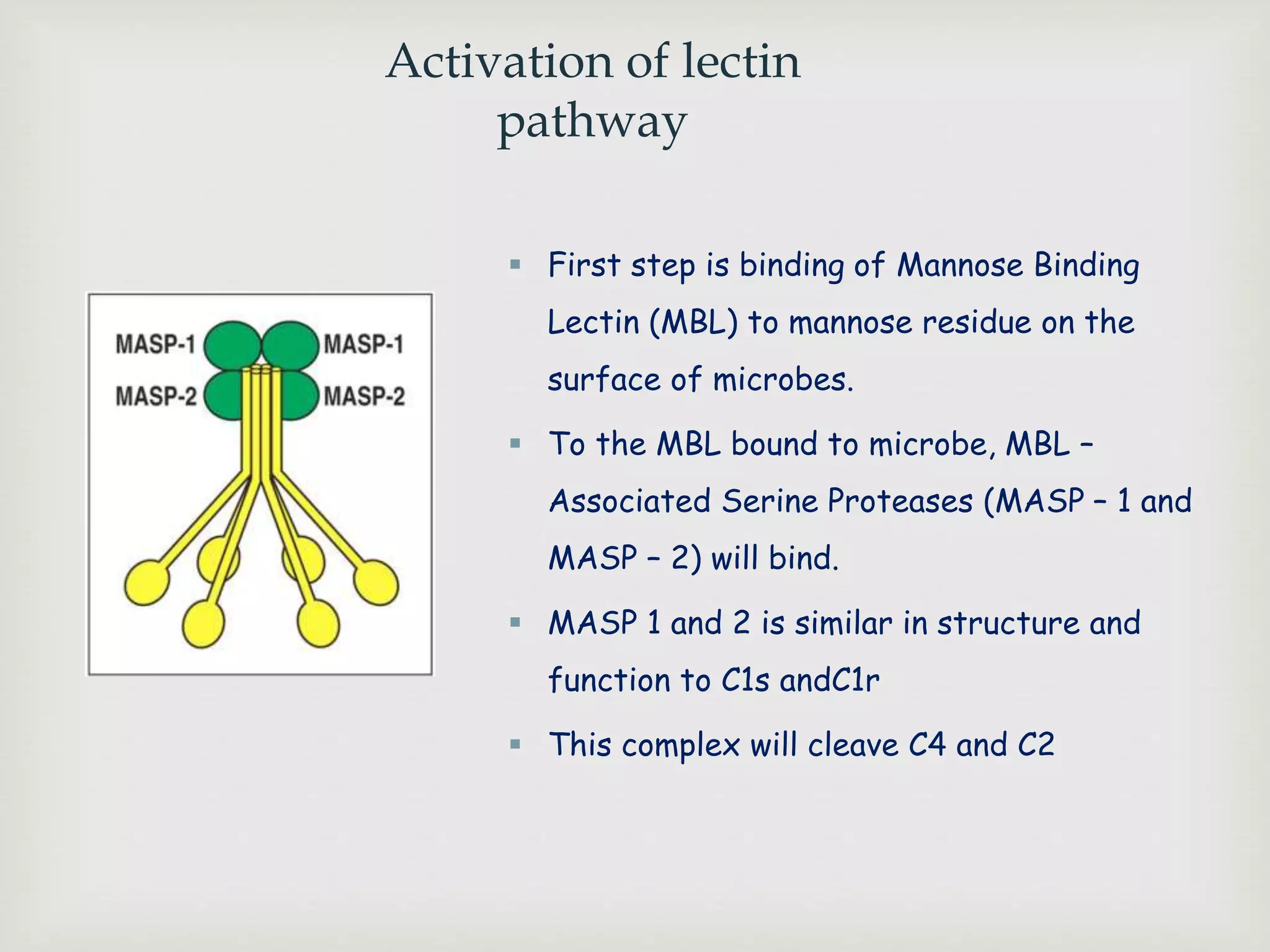
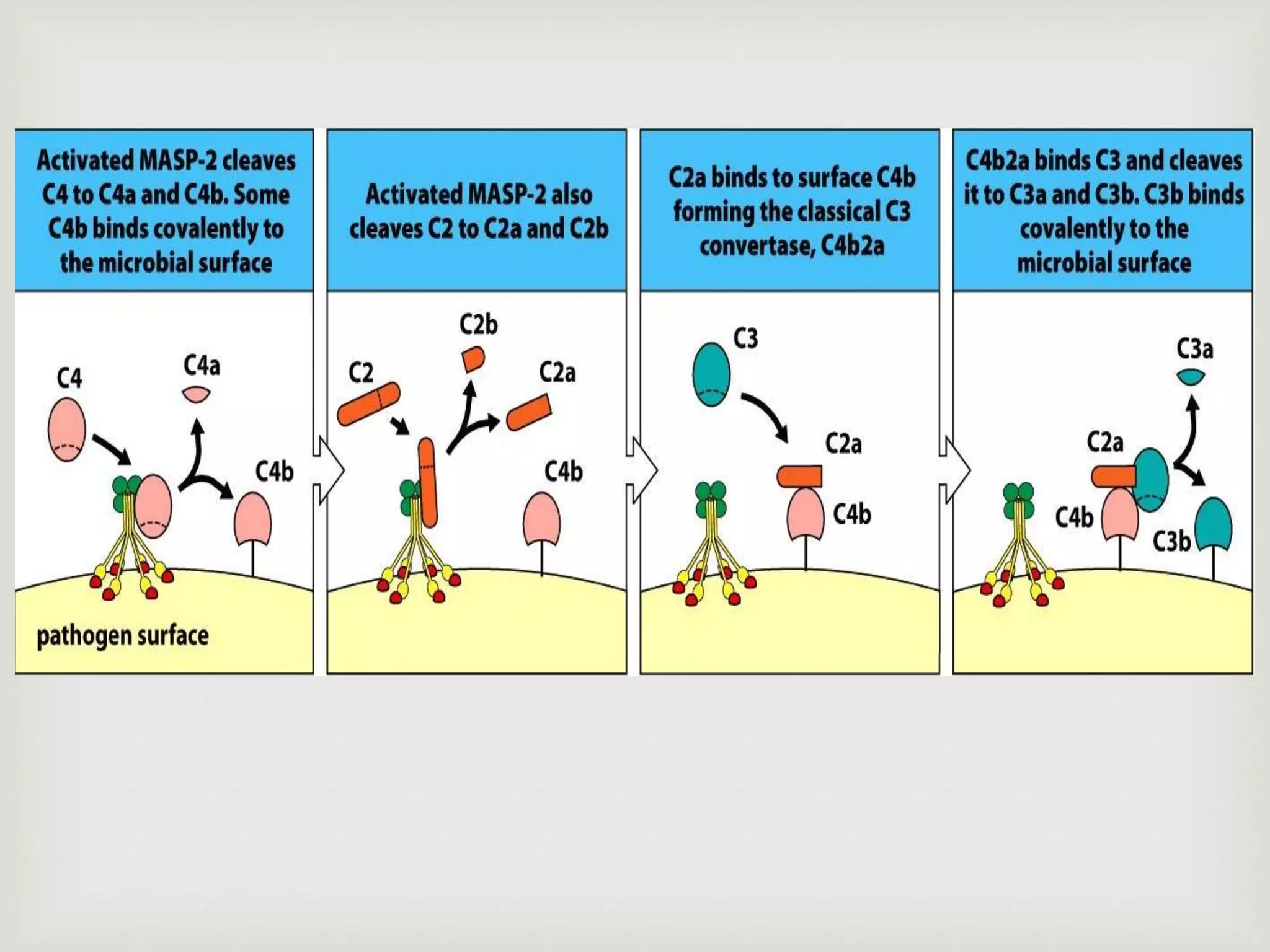
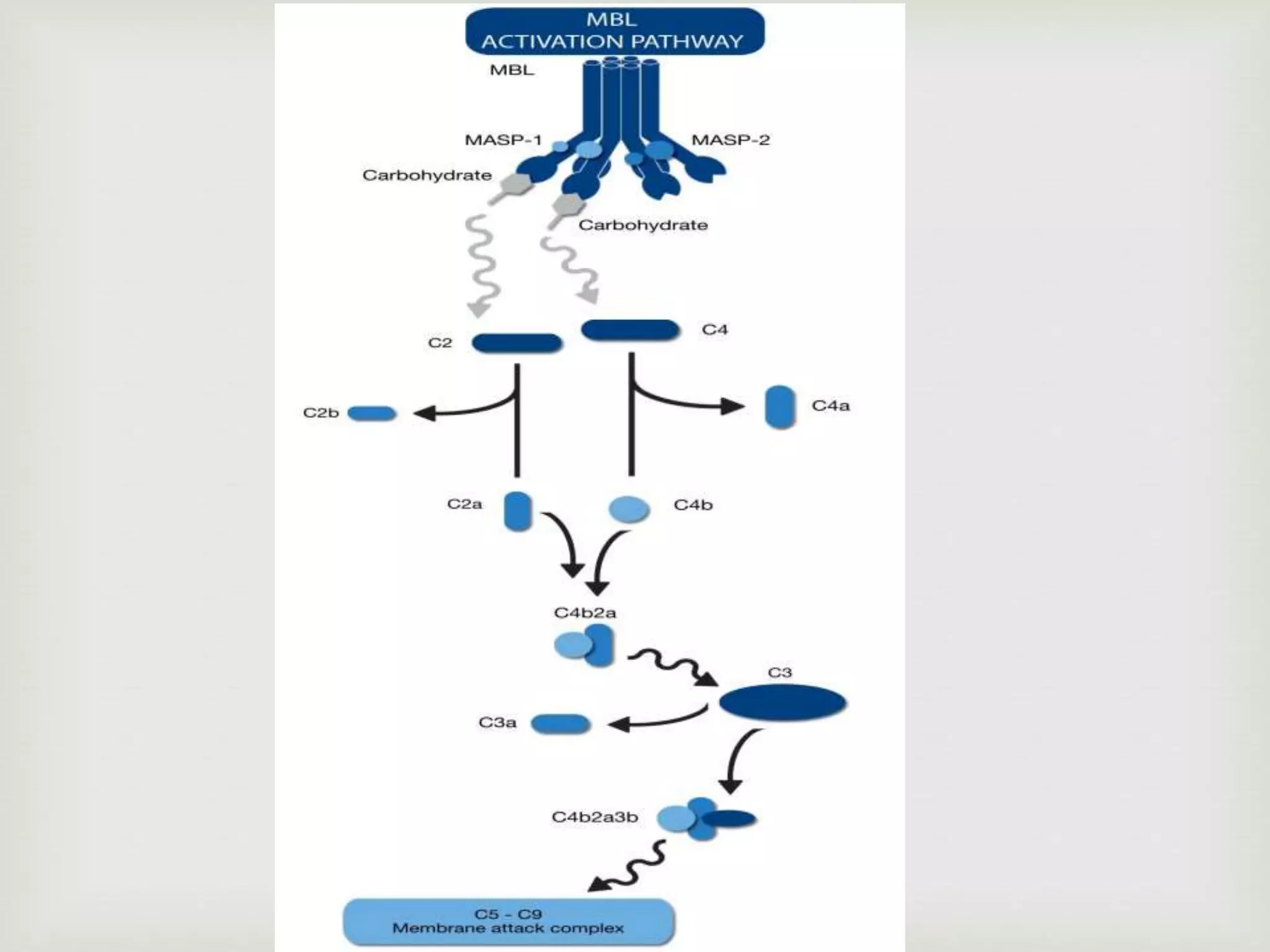
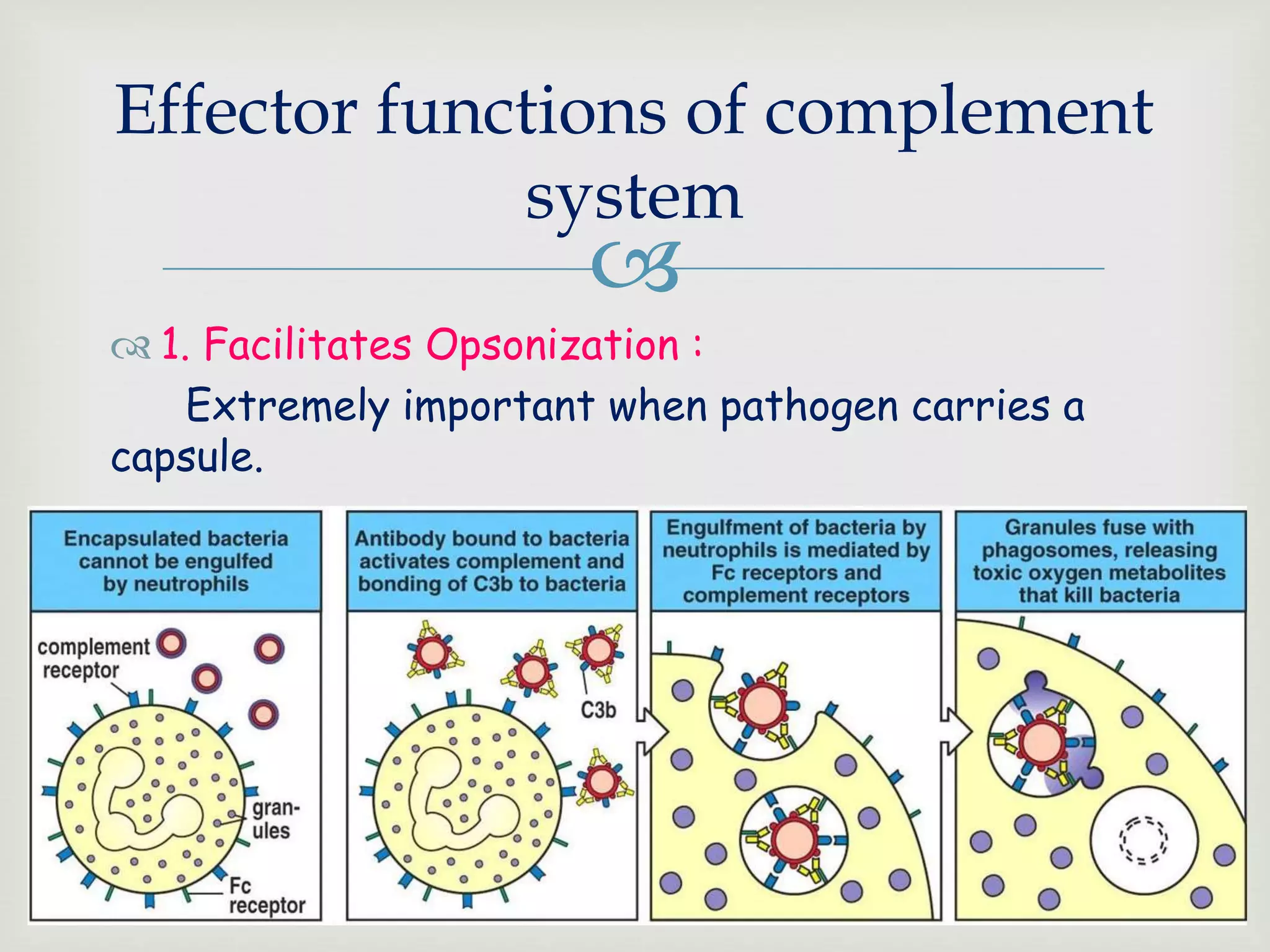
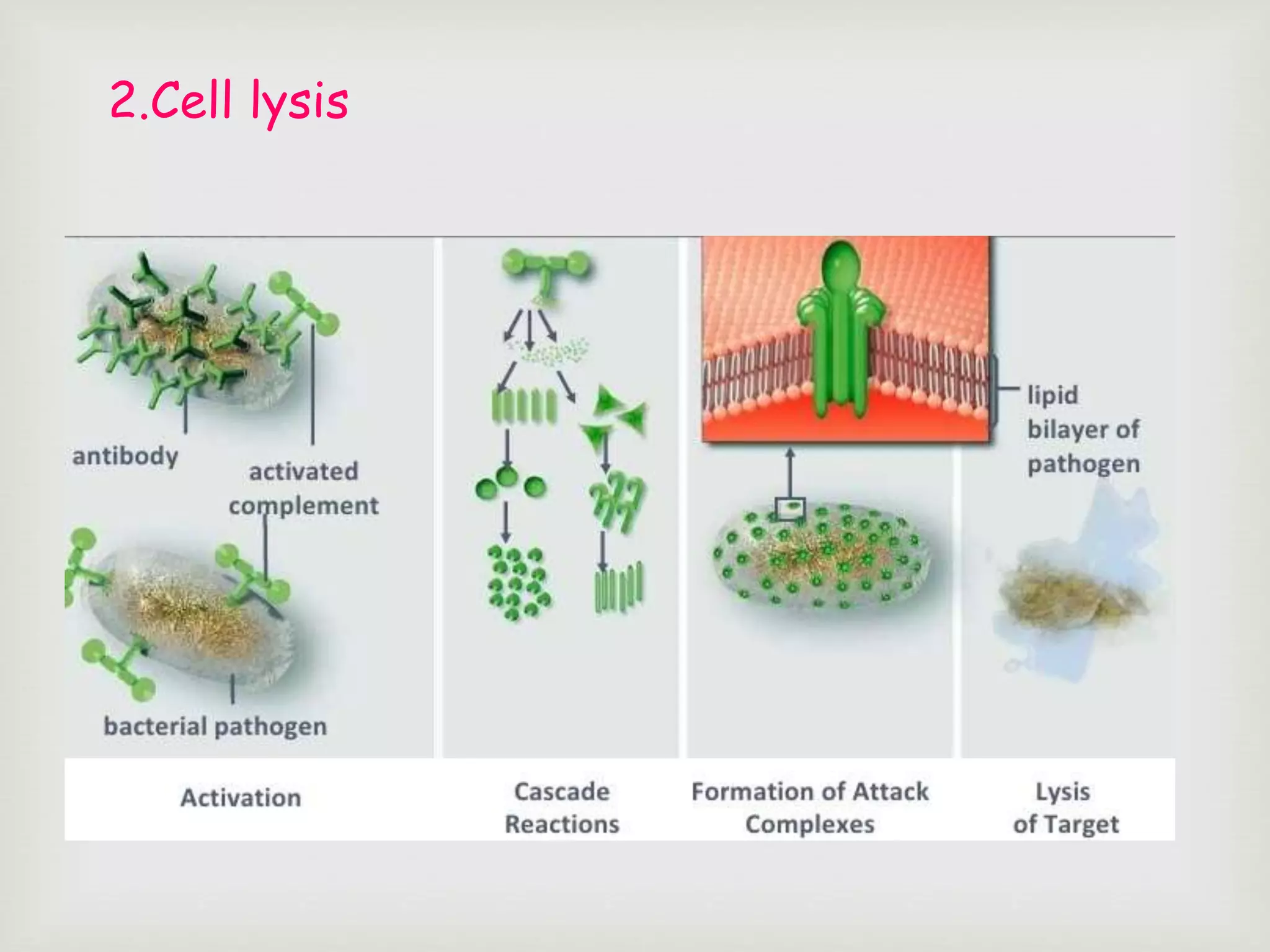
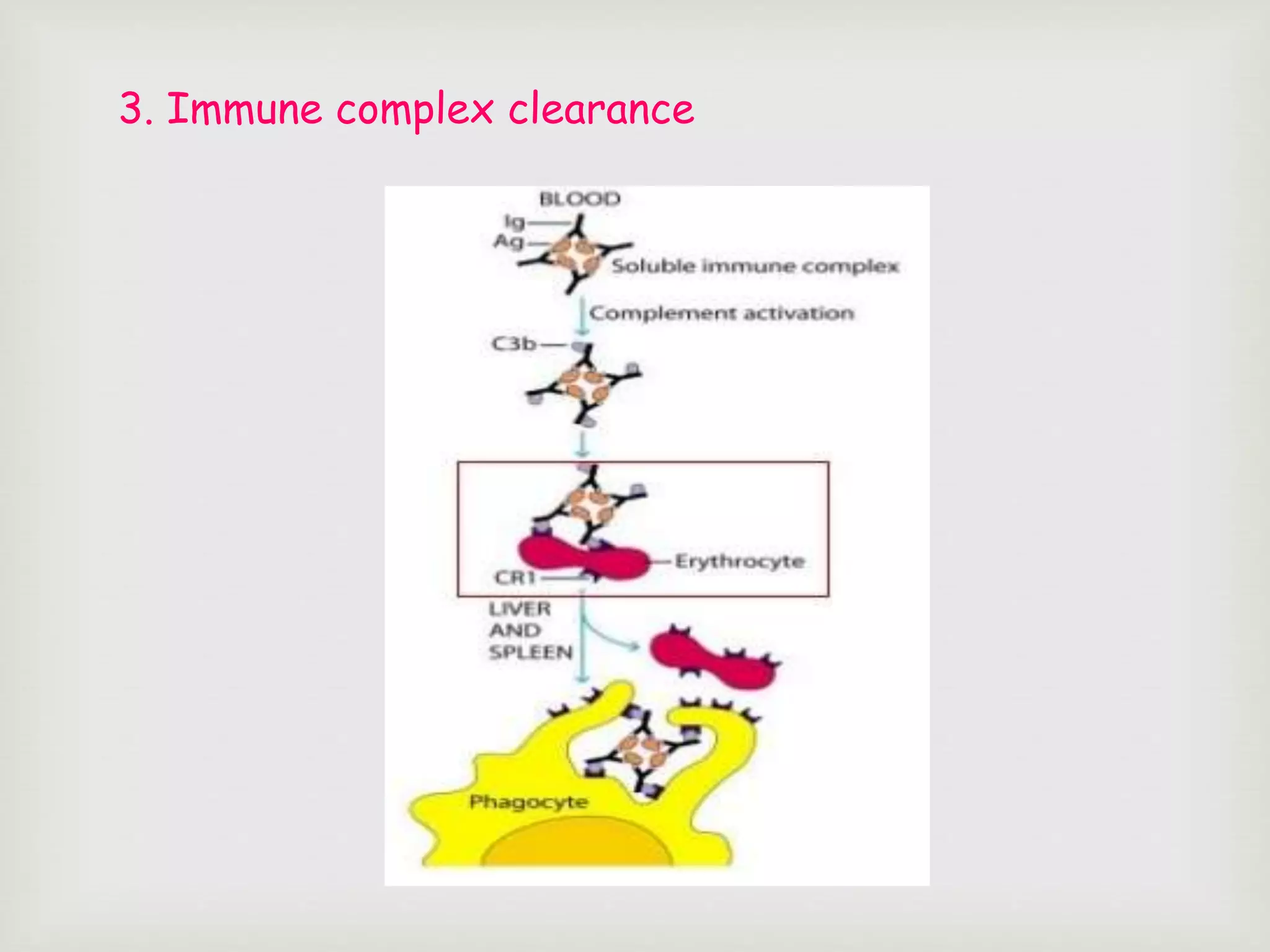
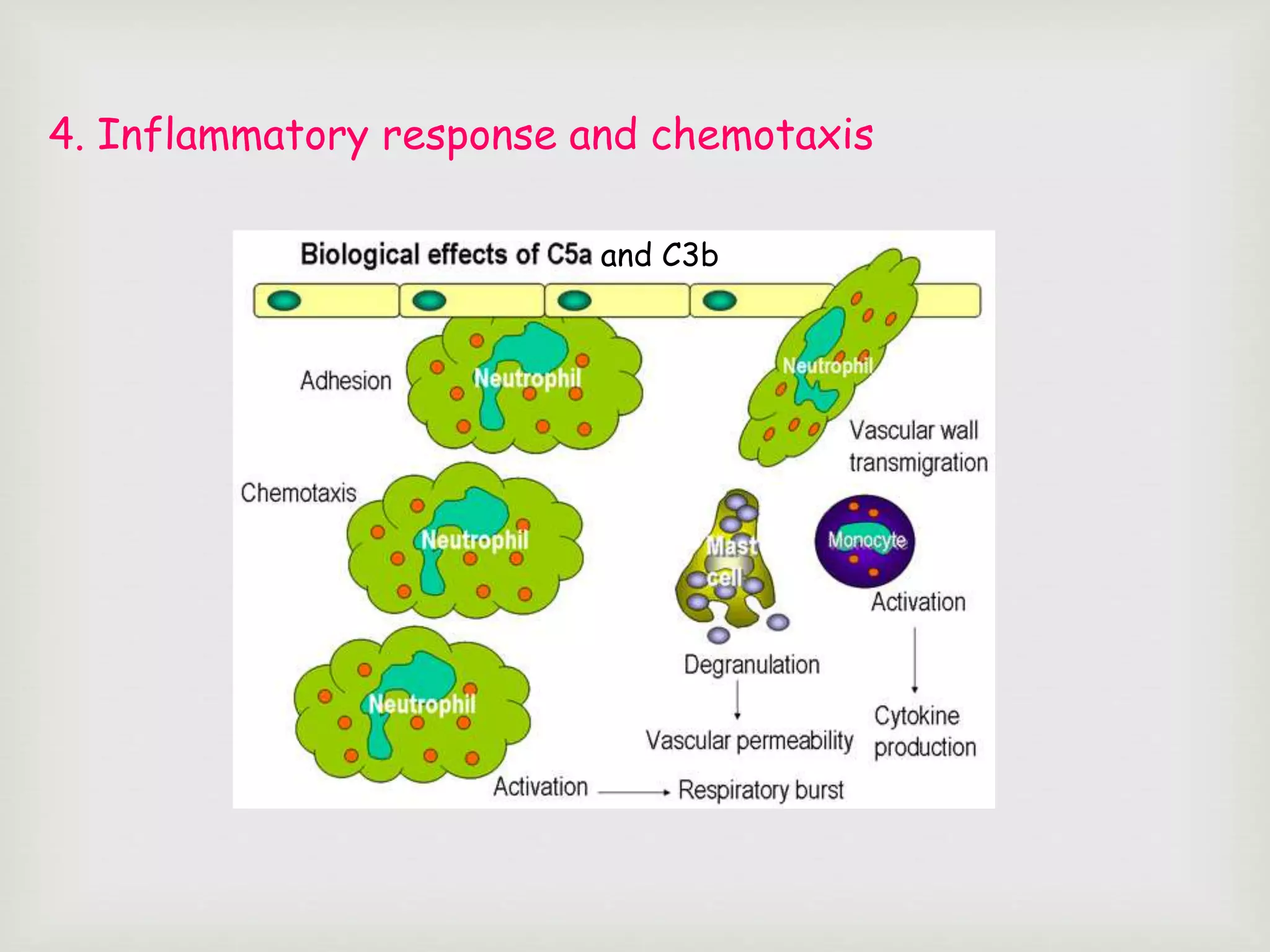
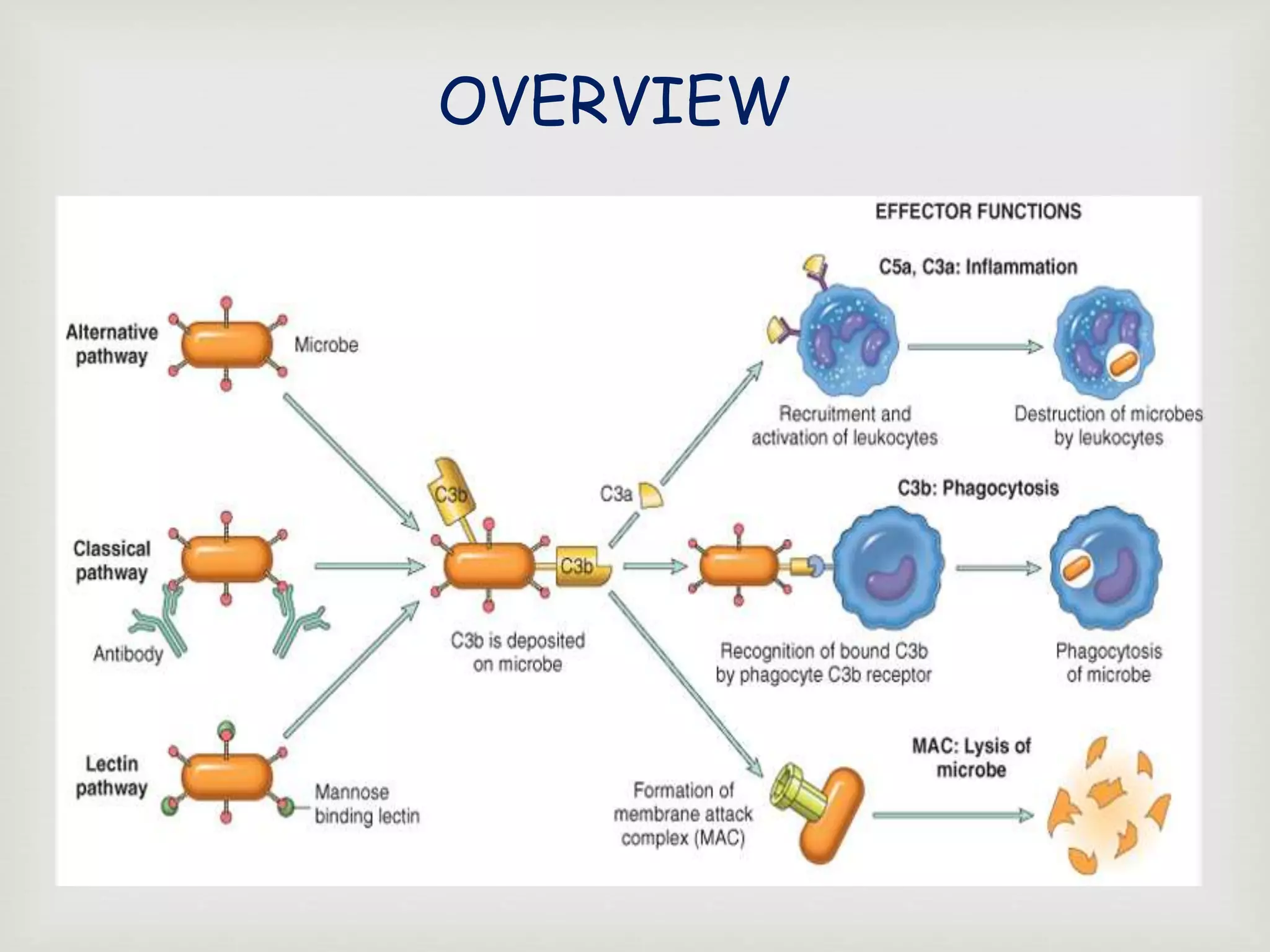
The document presents an overview of the complement system, detailing its history, components, and functions in the immune response. It explains the pathways of activation, including the classical, alternative, and lectin pathways, and highlights the roles of complement proteins in opsonization, inflammation, and pathogen clearance. The complement system is vital for both innate and adaptive immunity, contributing significantly to the body's defense mechanisms against infections.