0% found this document useful (0 votes)
1K views1 pageNeutralization and Hydrolysis Worksheet
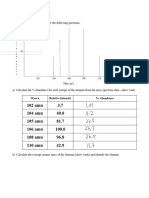
The document discusses neutralization and hydrolysis reactions involving acids and bases to form salts. It notes that strong acid + strong base reactions form neutral salts, strong acid + weak base reactions form acidic salts, and weak acid + strong base reactions form basic salts. A table is included that asks the reader to identify the parent acid, parent base, and type of solution (acidic, basic, or neutral) for various salt compounds.
Uploaded by
LiviaAsriCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views1 pageNeutralization and Hydrolysis Worksheet
The document discusses neutralization and hydrolysis reactions involving acids and bases to form salts. It notes that strong acid + strong base reactions form neutral salts, strong acid + weak base reactions form acidic salts, and weak acid + strong base reactions form basic salts. A table is included that asks the reader to identify the parent acid, parent base, and type of solution (acidic, basic, or neutral) for various salt compounds.
Uploaded by
LiviaAsriCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOC, PDF, TXT or read online on Scribd
/ 1