Download to read offline

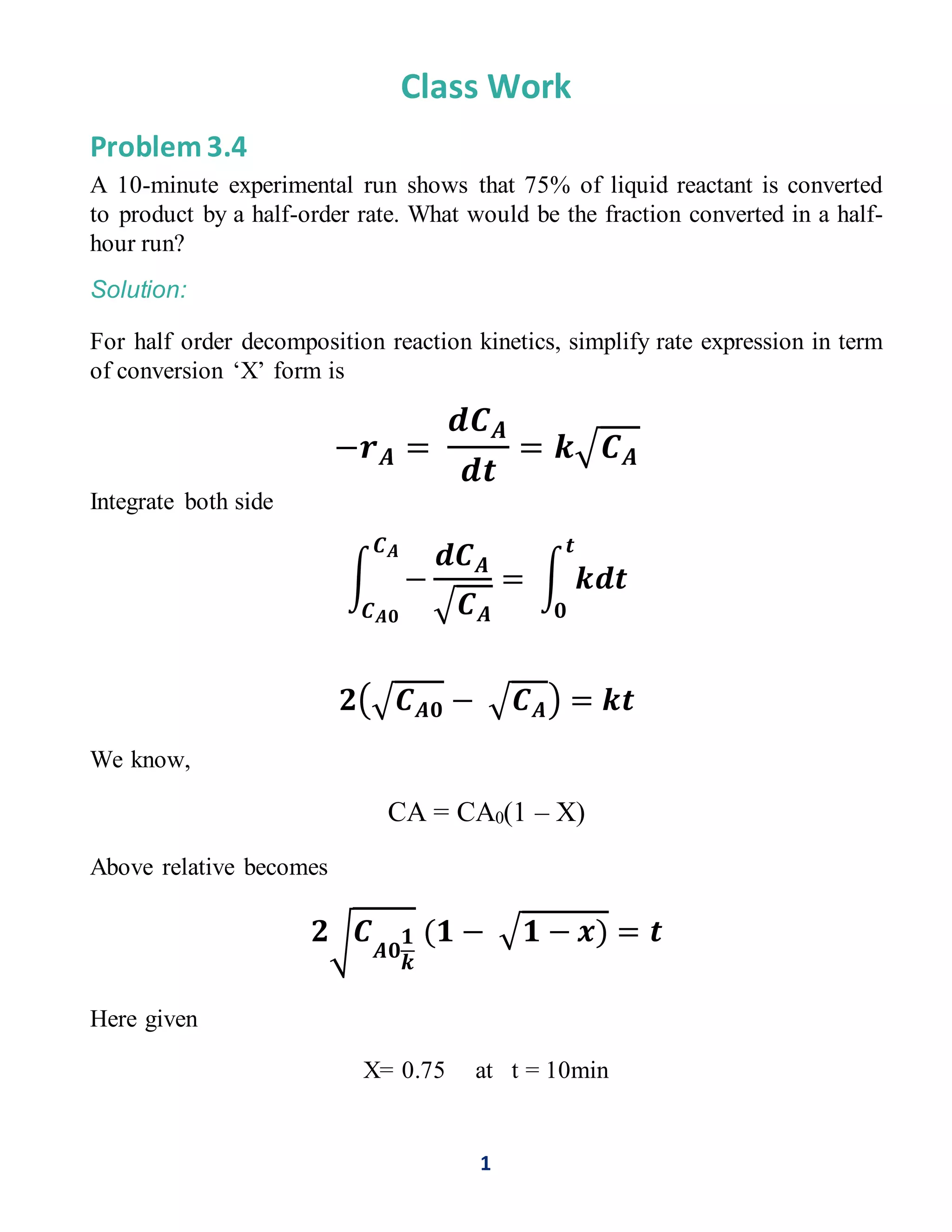


The document discusses a chemical reaction engineering problem involving a half-order rate reaction. It calculates the fraction of liquid reactant converted to product over a specified time, concluding that a 10-minute run results in 75% conversion. The analysis shows the same conversion percentage applies to a half-hour run.


