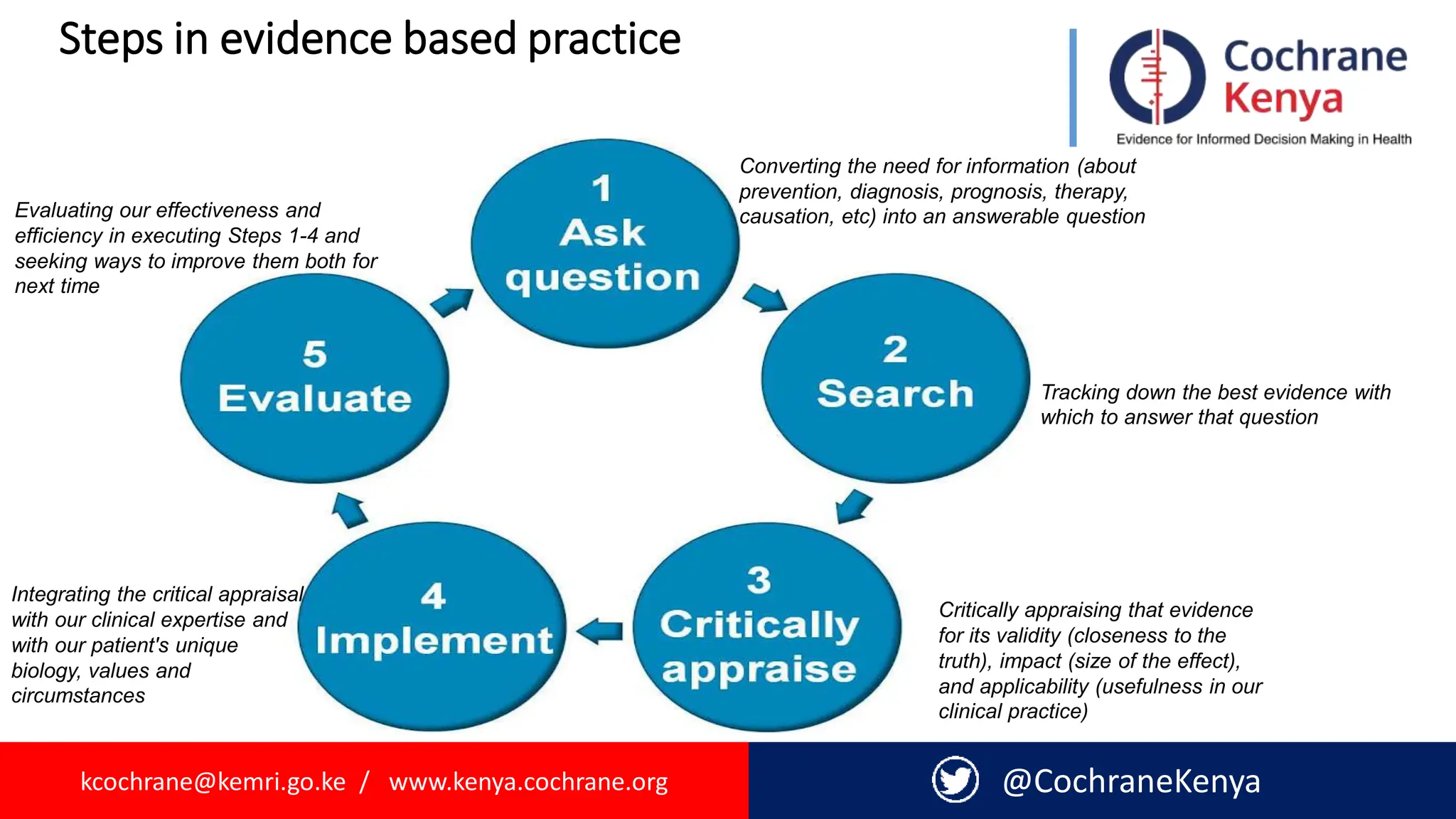
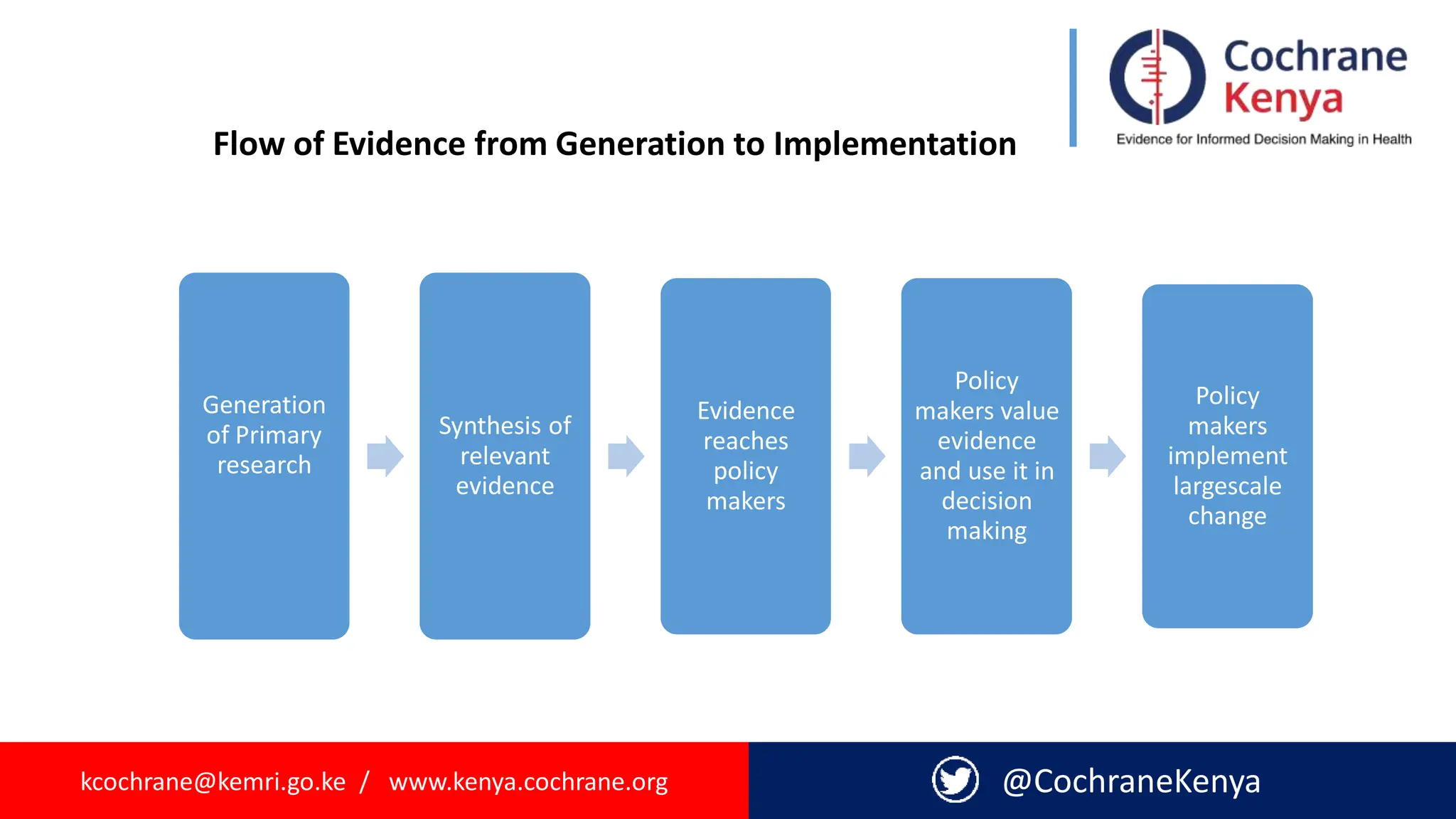
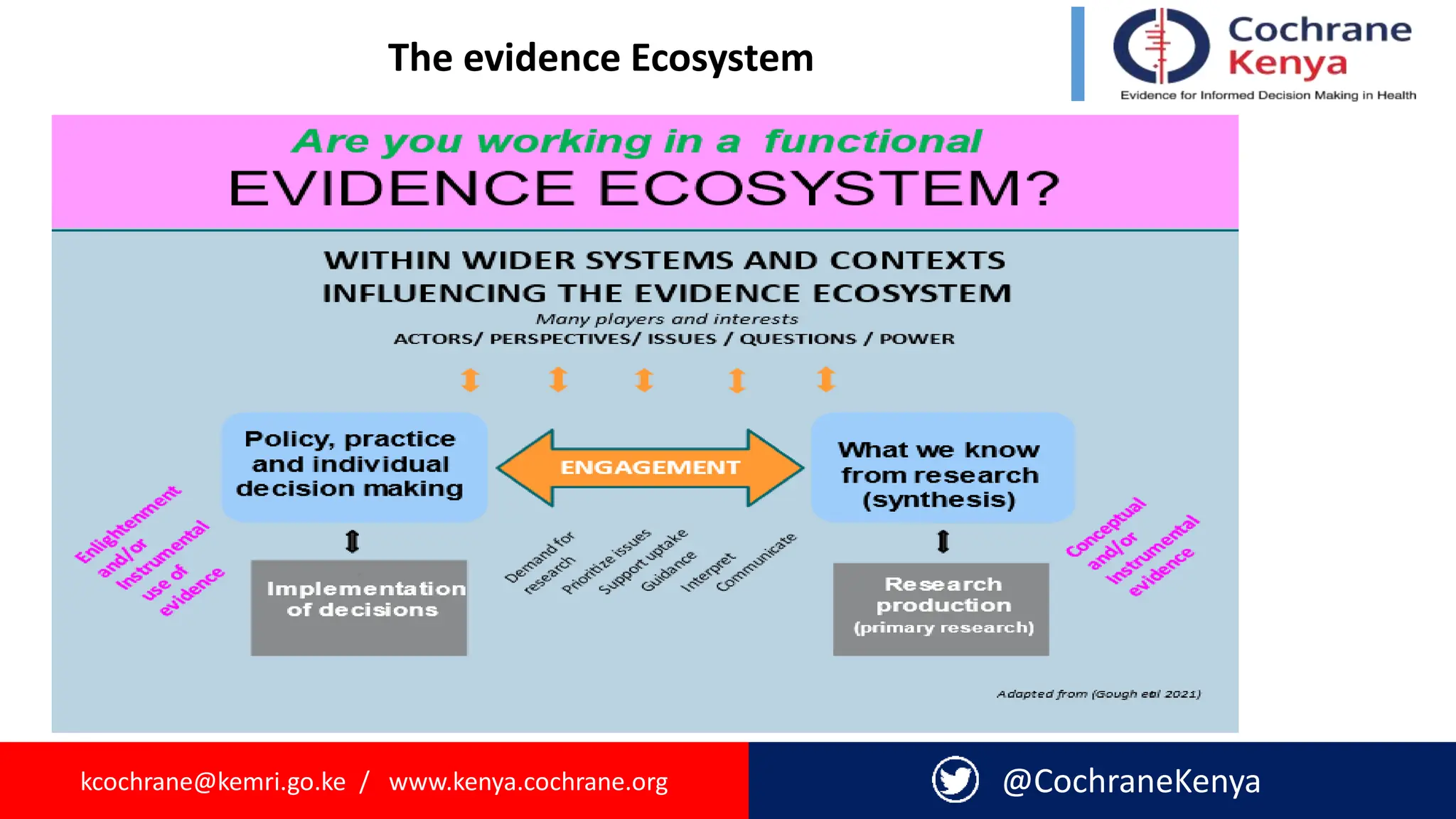
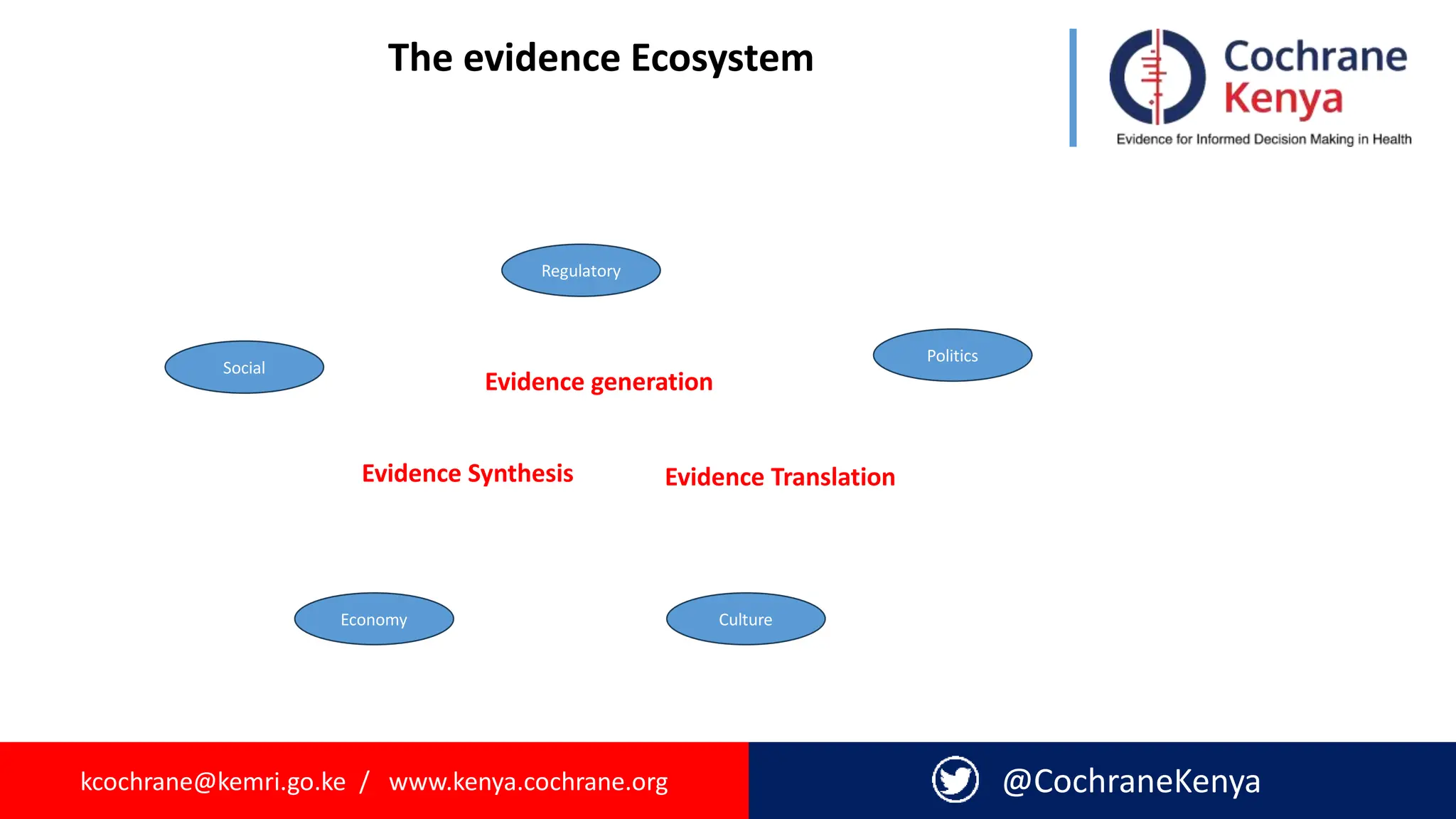
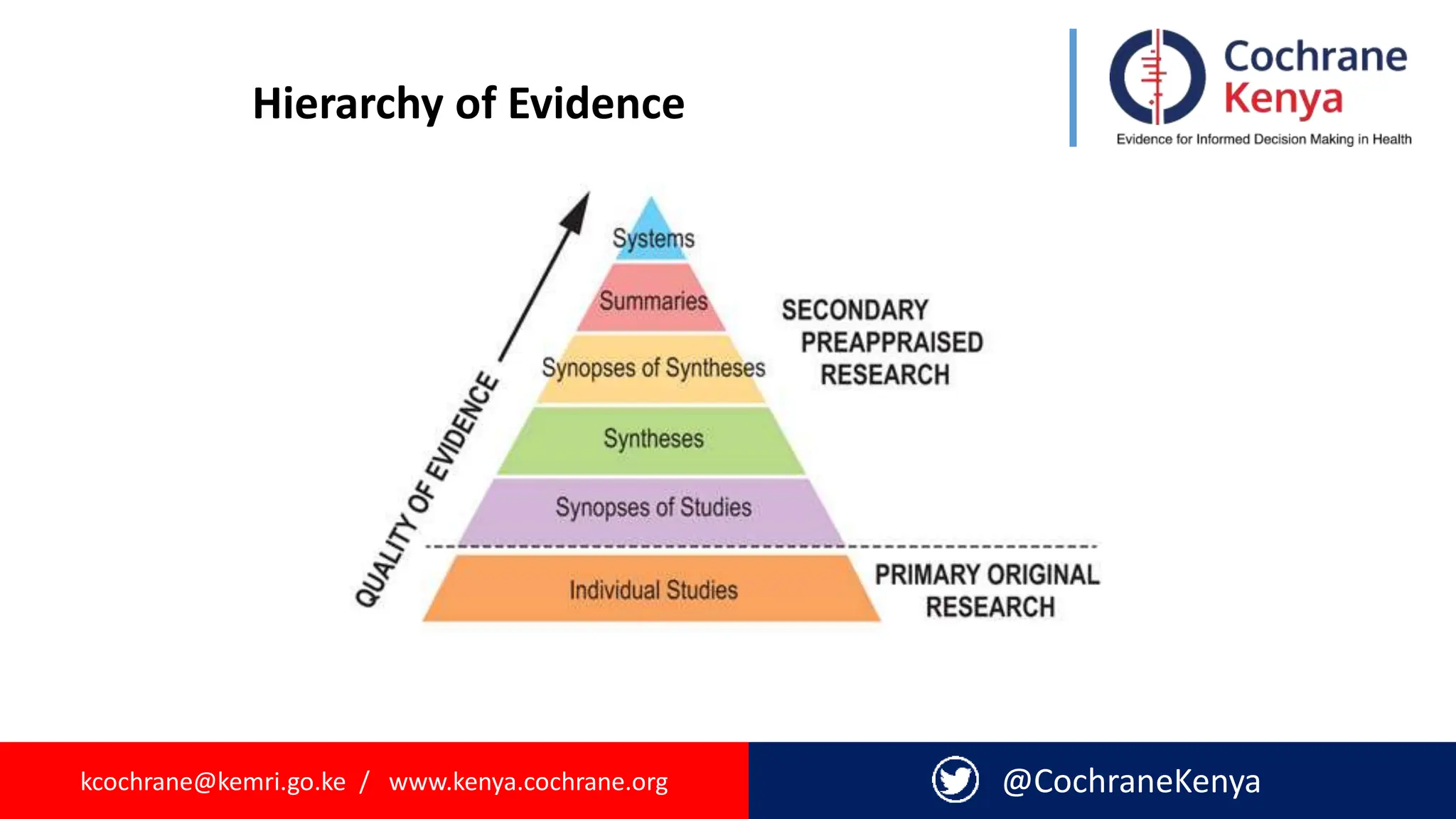
The document provides an overview of evidence-informed decision making and systematic reviews, emphasizing the integration of best research evidence with clinical expertise and patient values. It outlines the steps in evidence-based practice, the importance of evidence synthesis, and various types of reviews, including narrative, scoping, rapid, and systematic reviews. Additionally, it discusses the systematic review process, including preparation, selection of studies, data extraction, and synthesis while highlighting the need for transparency and minimizing bias.