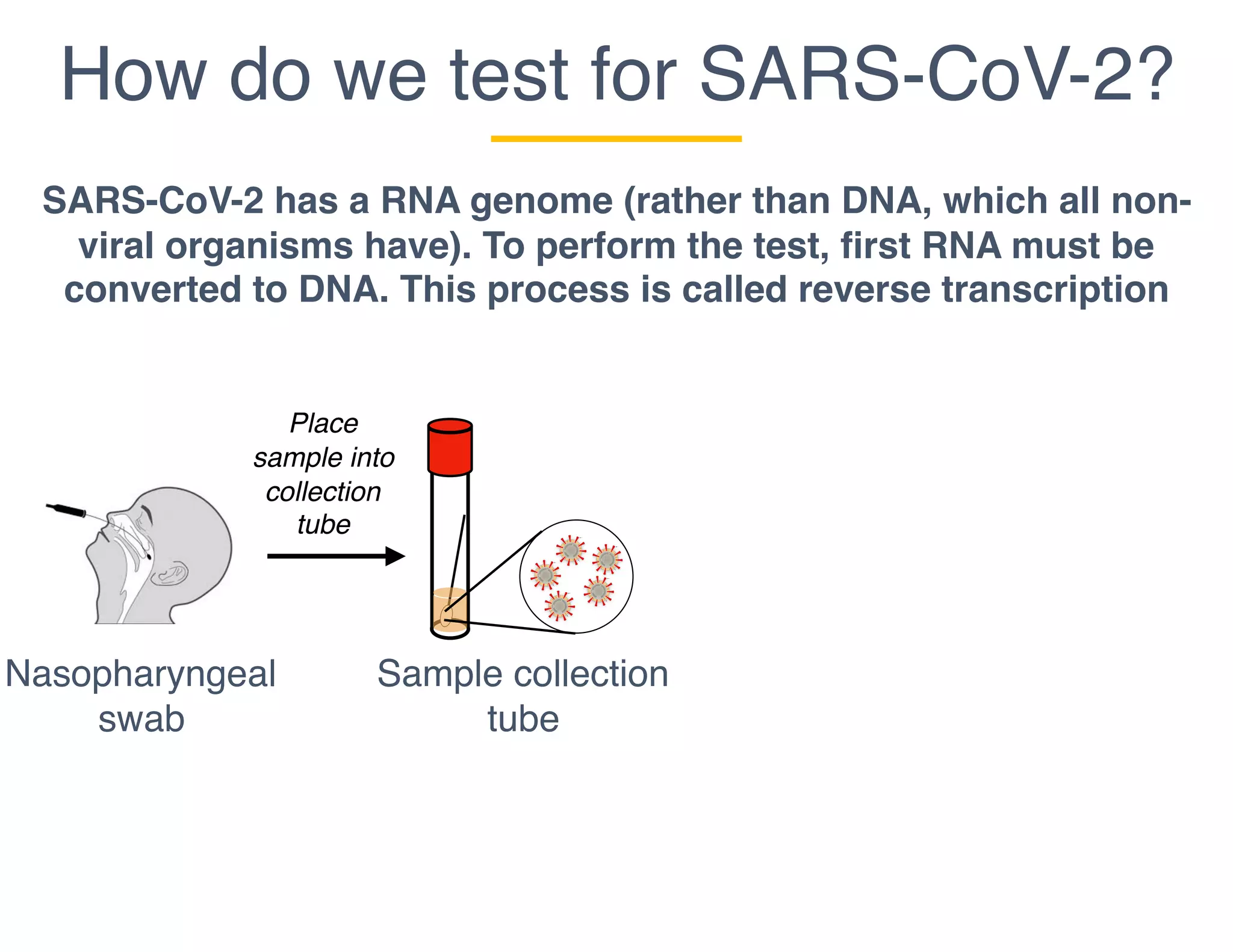
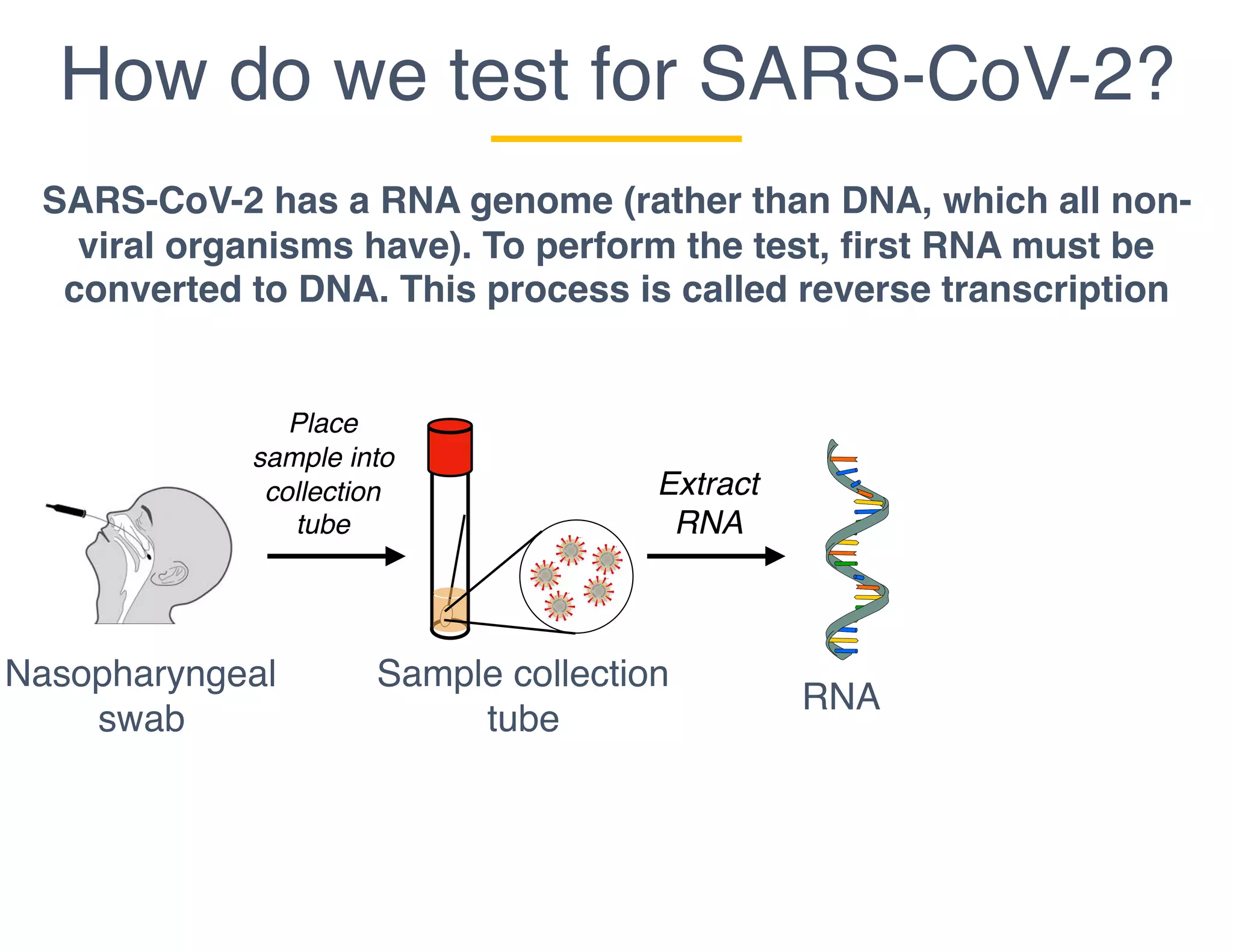
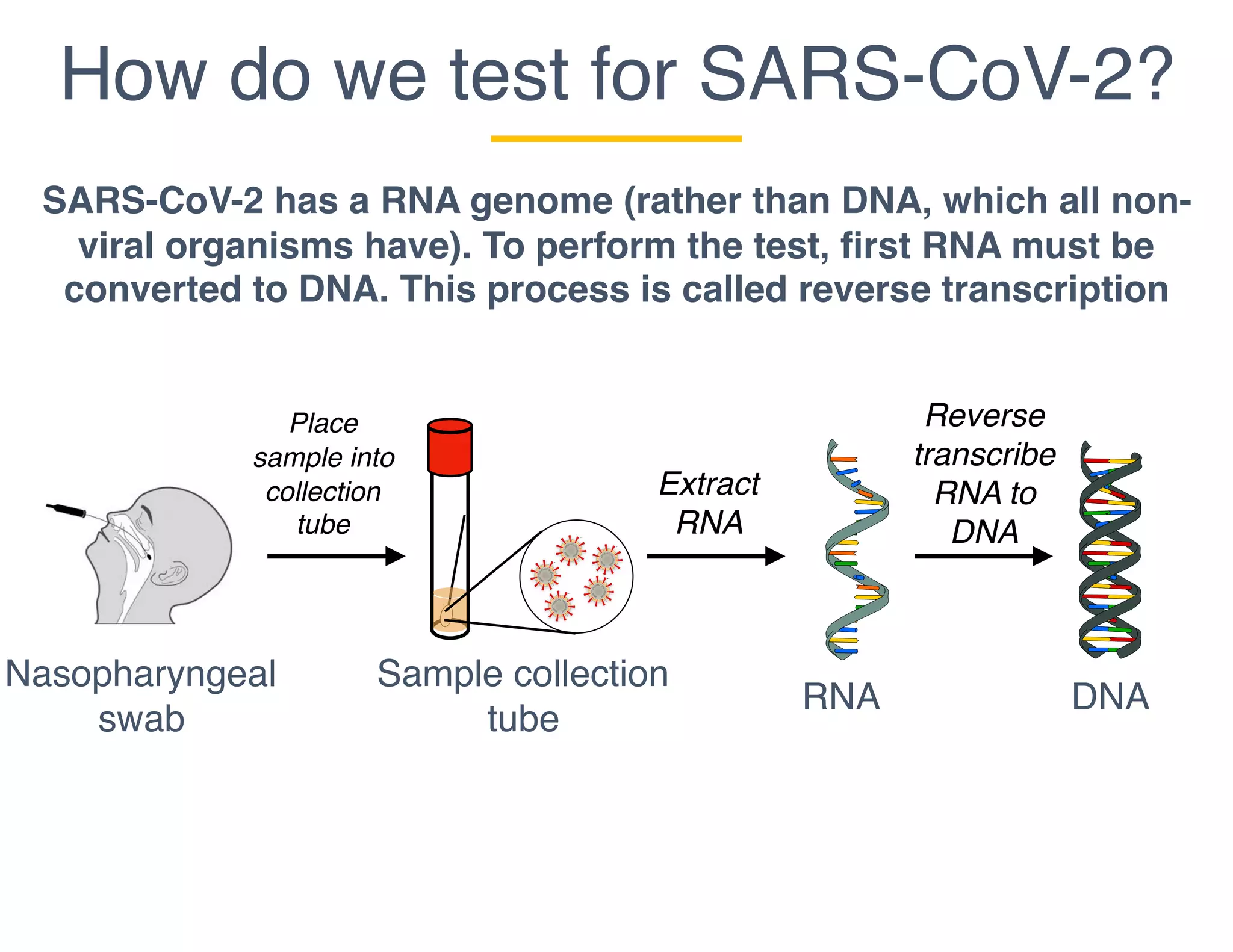
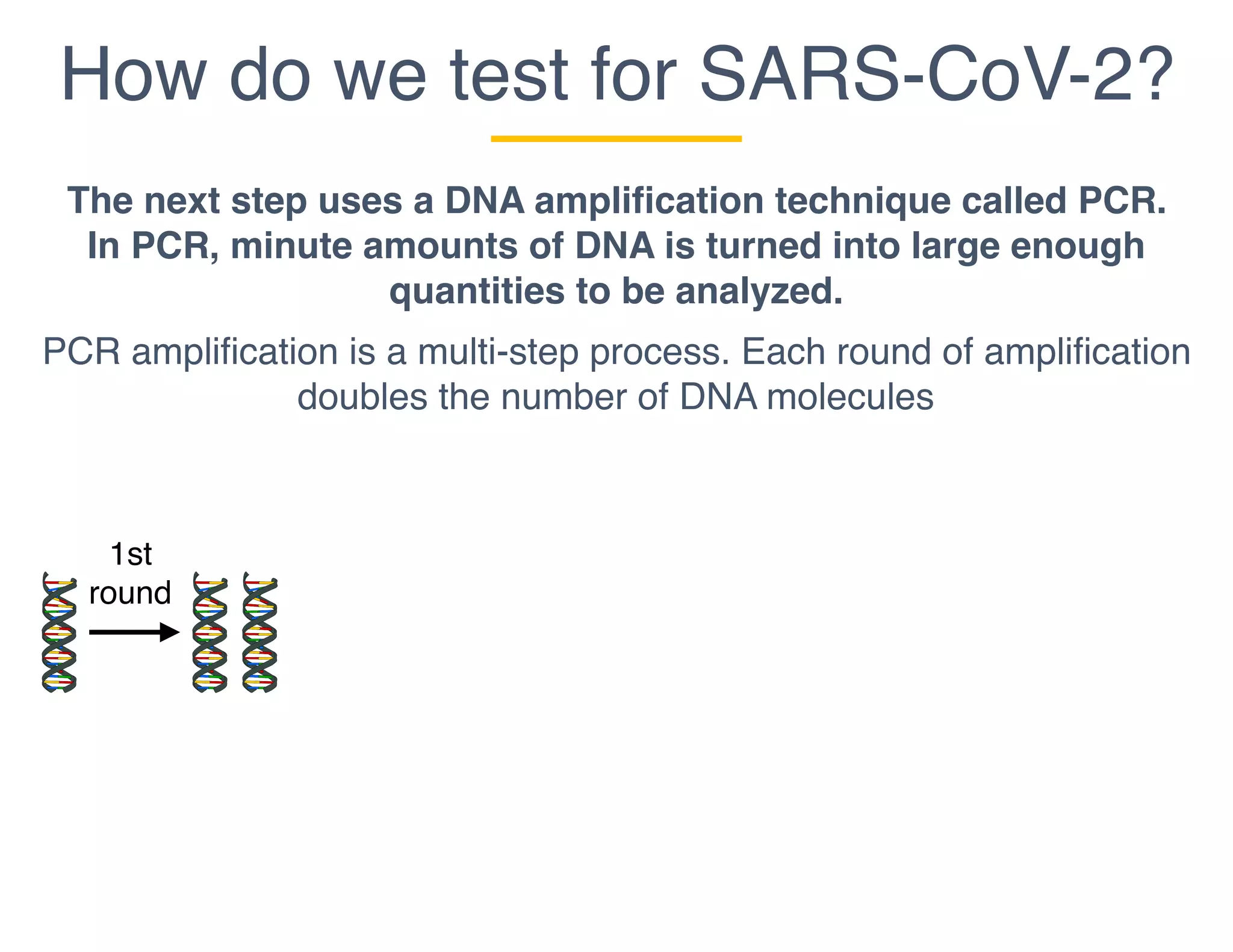
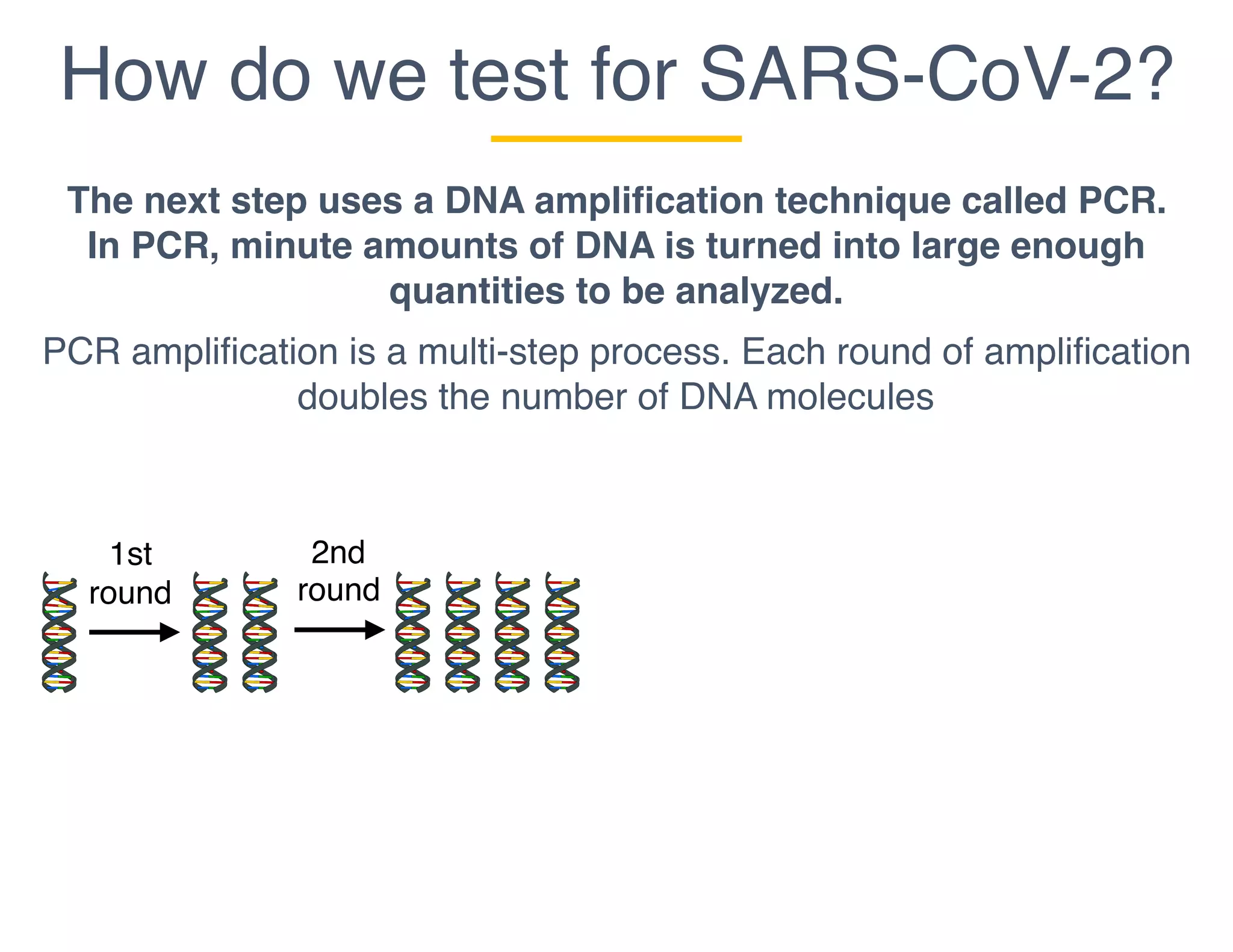
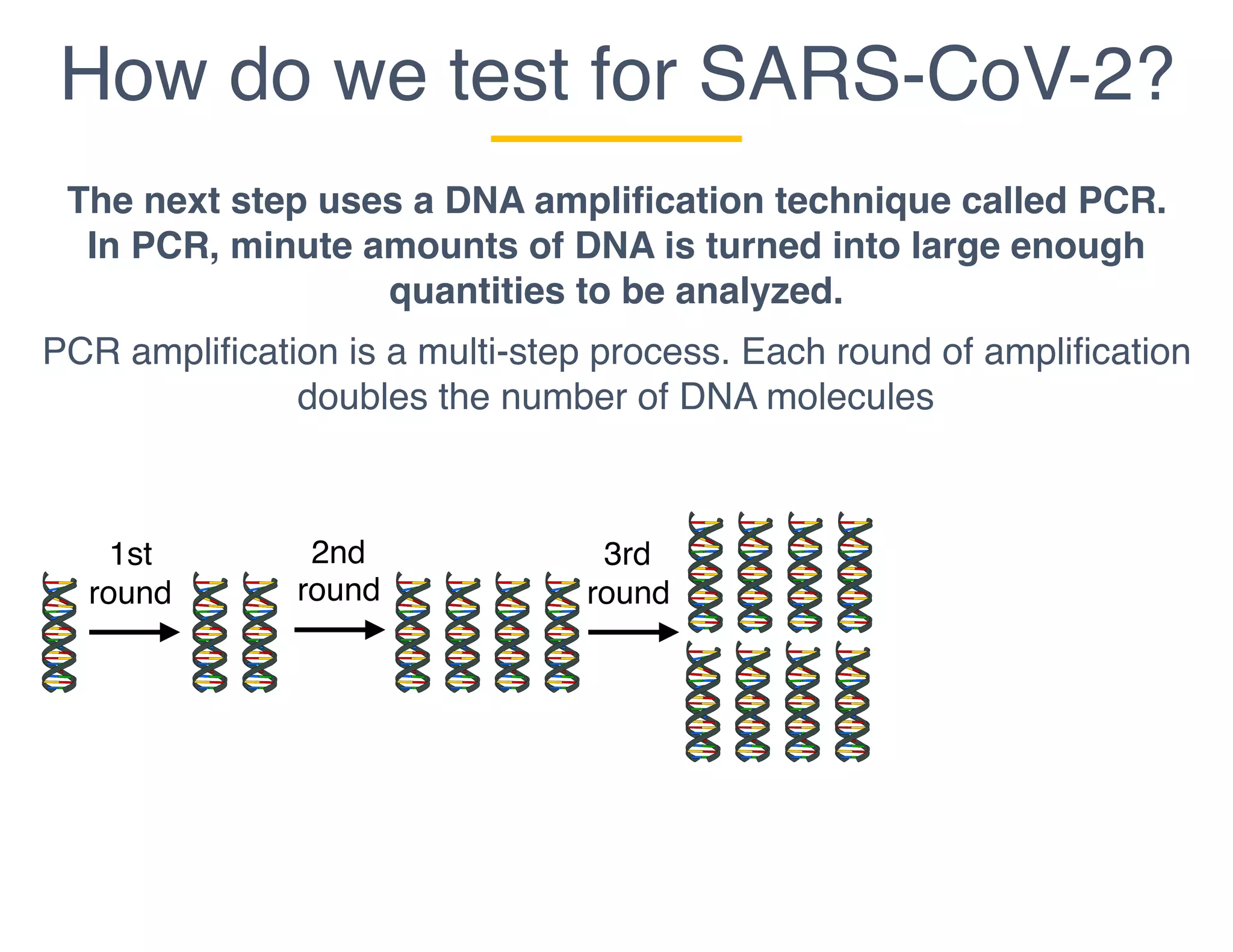
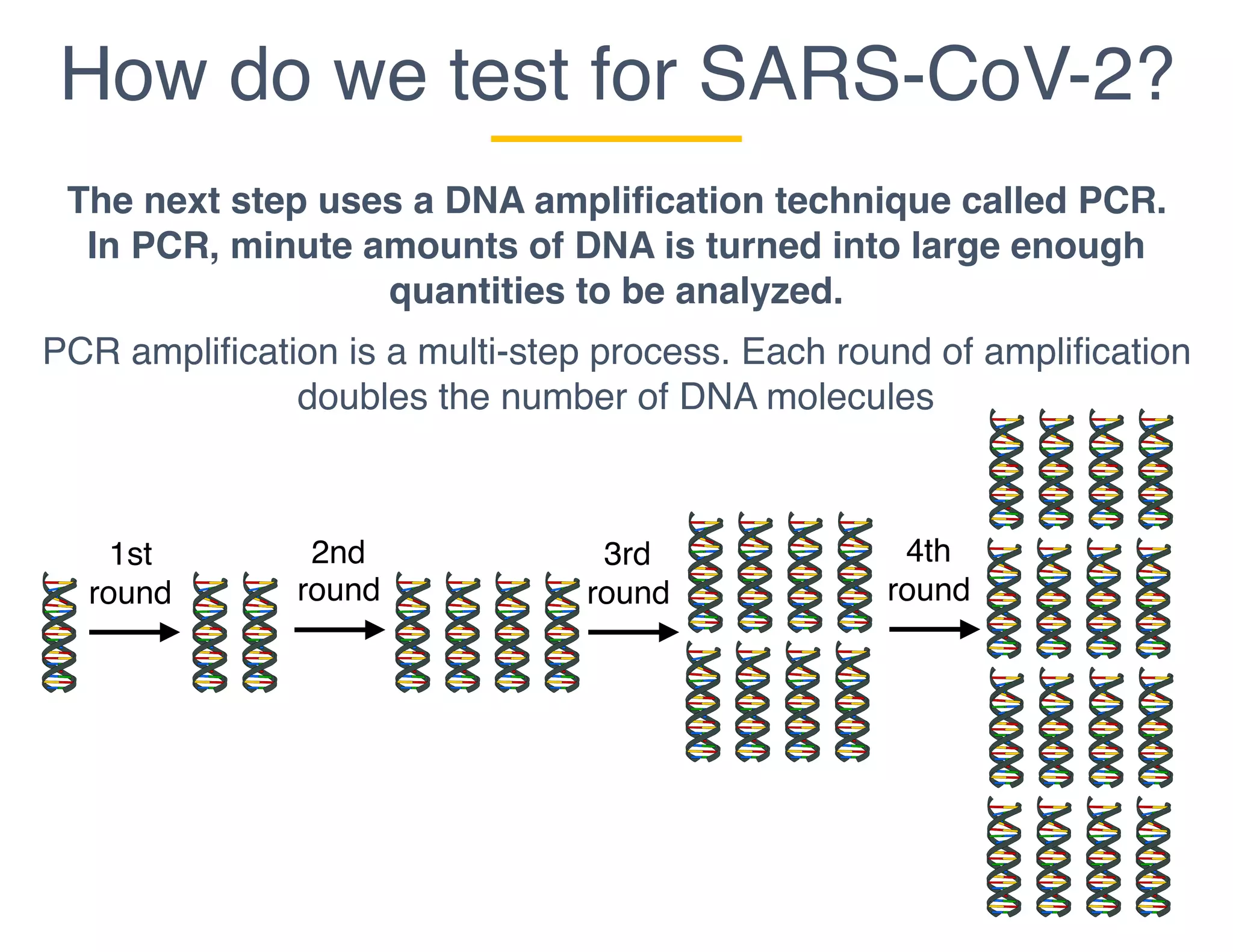
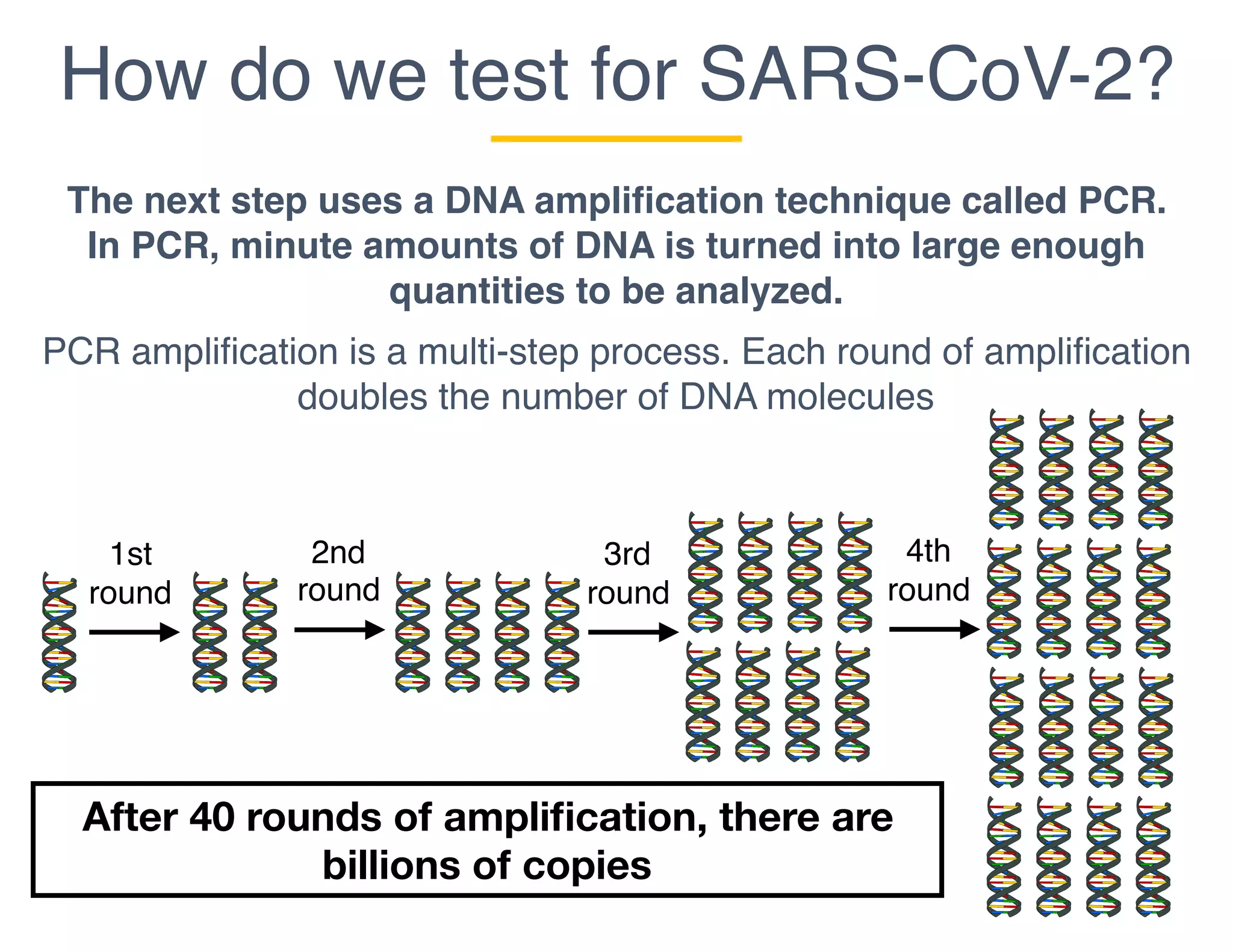
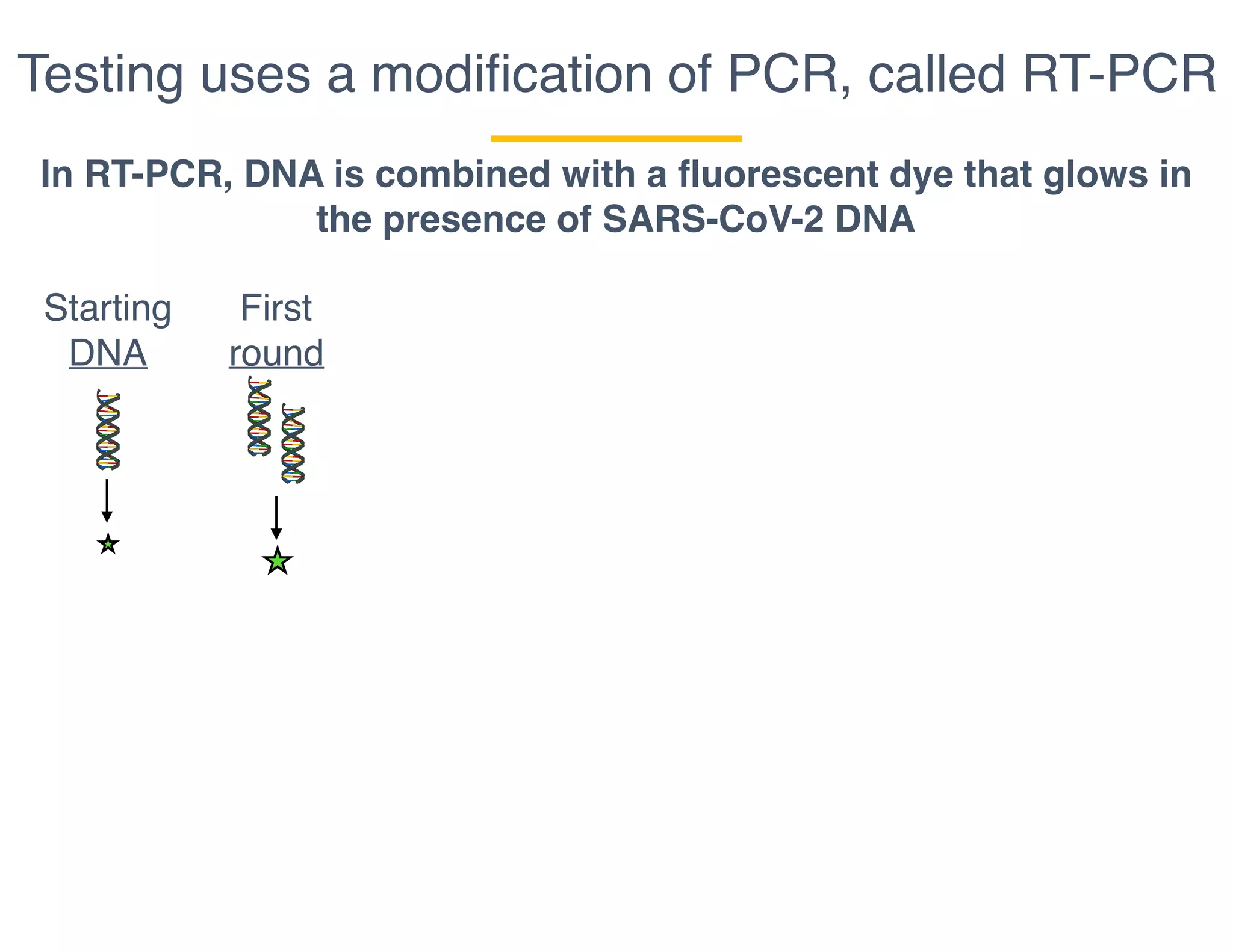
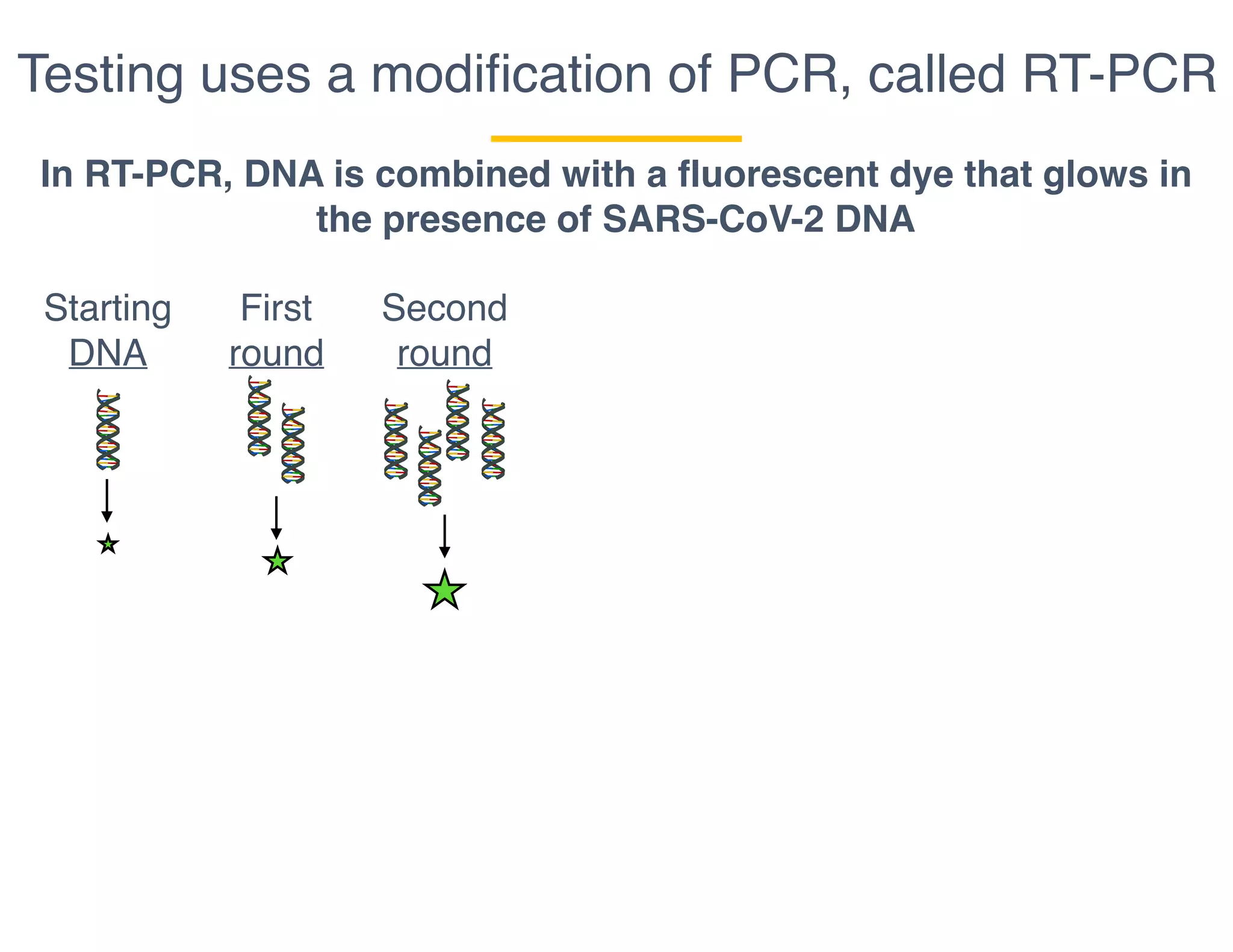
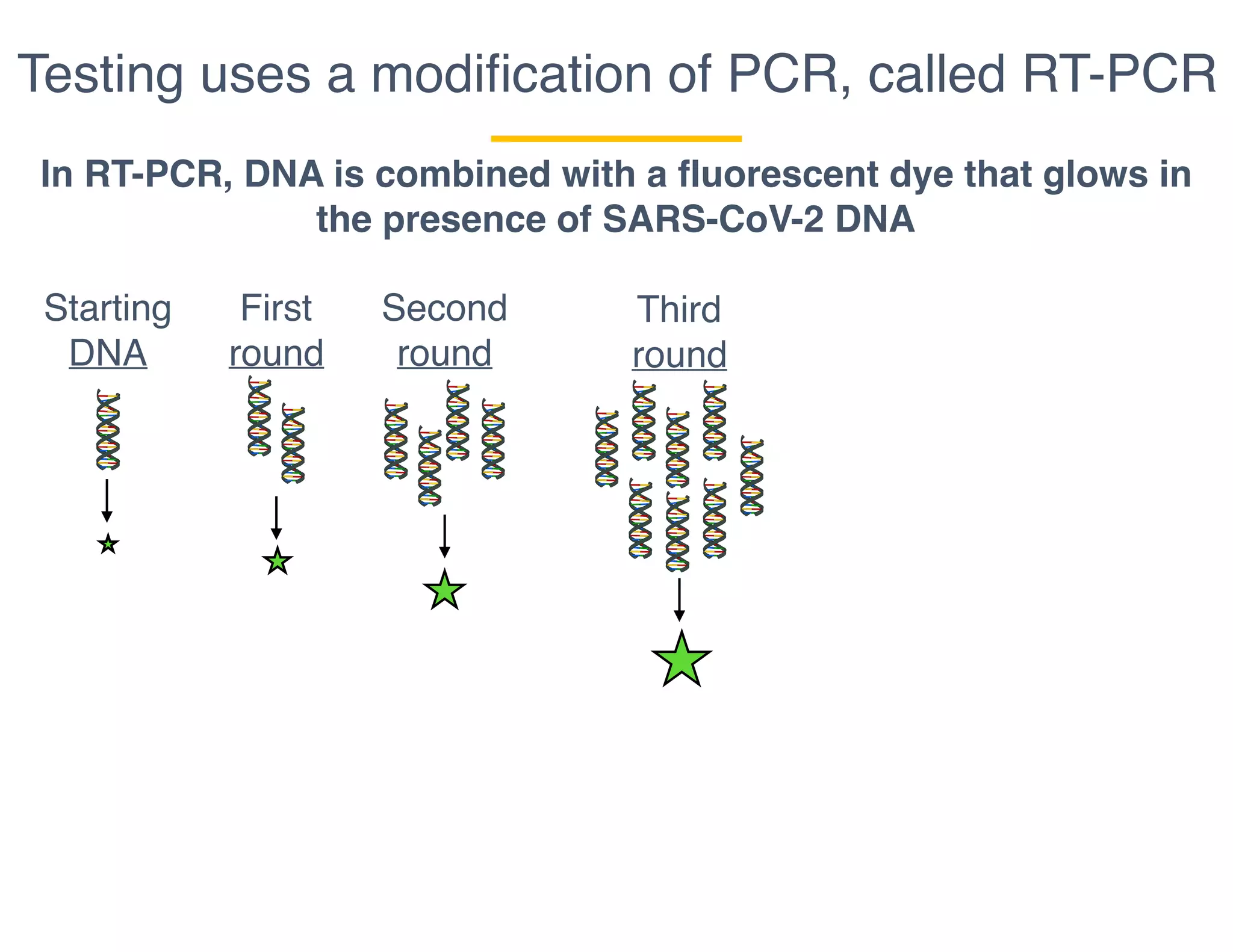
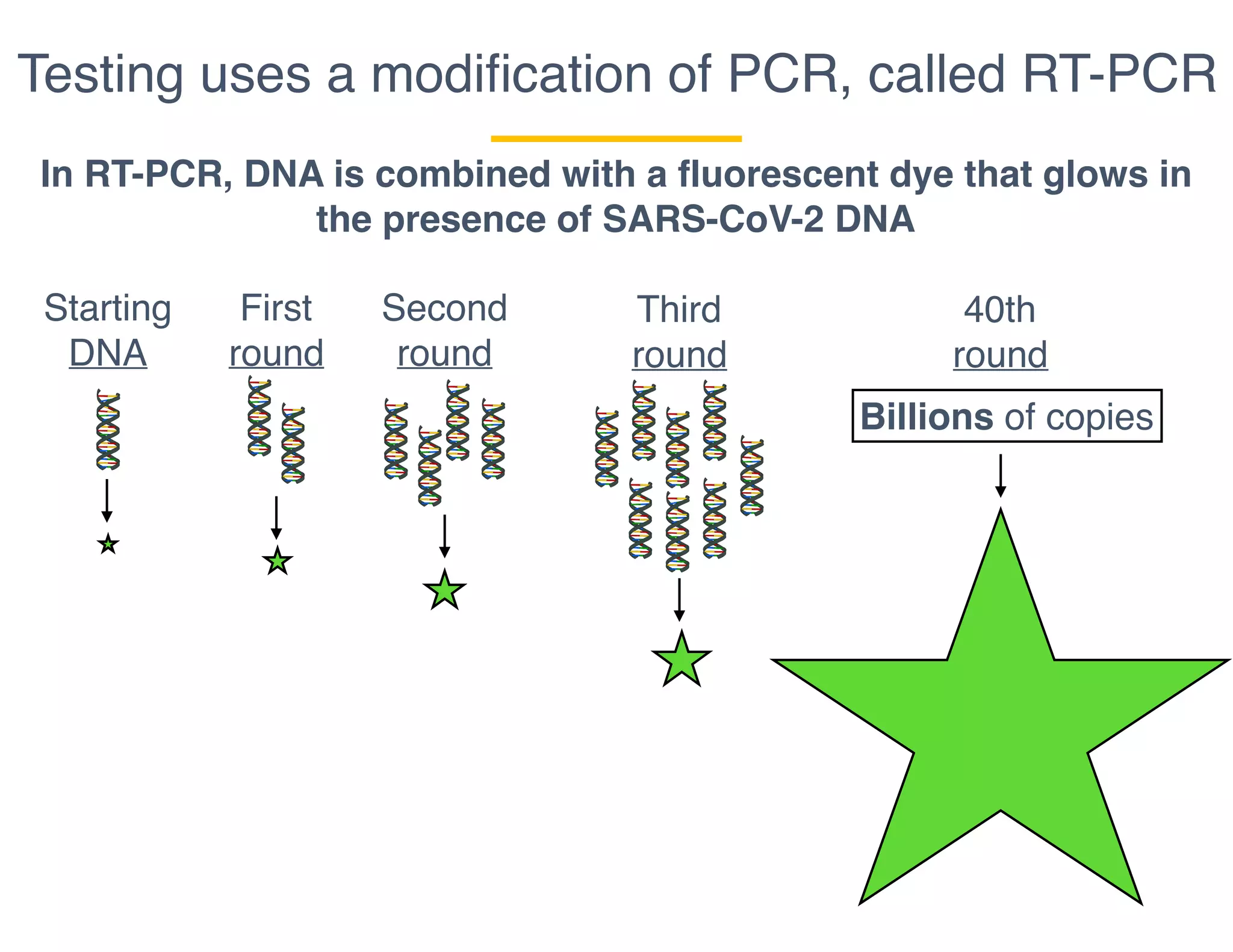
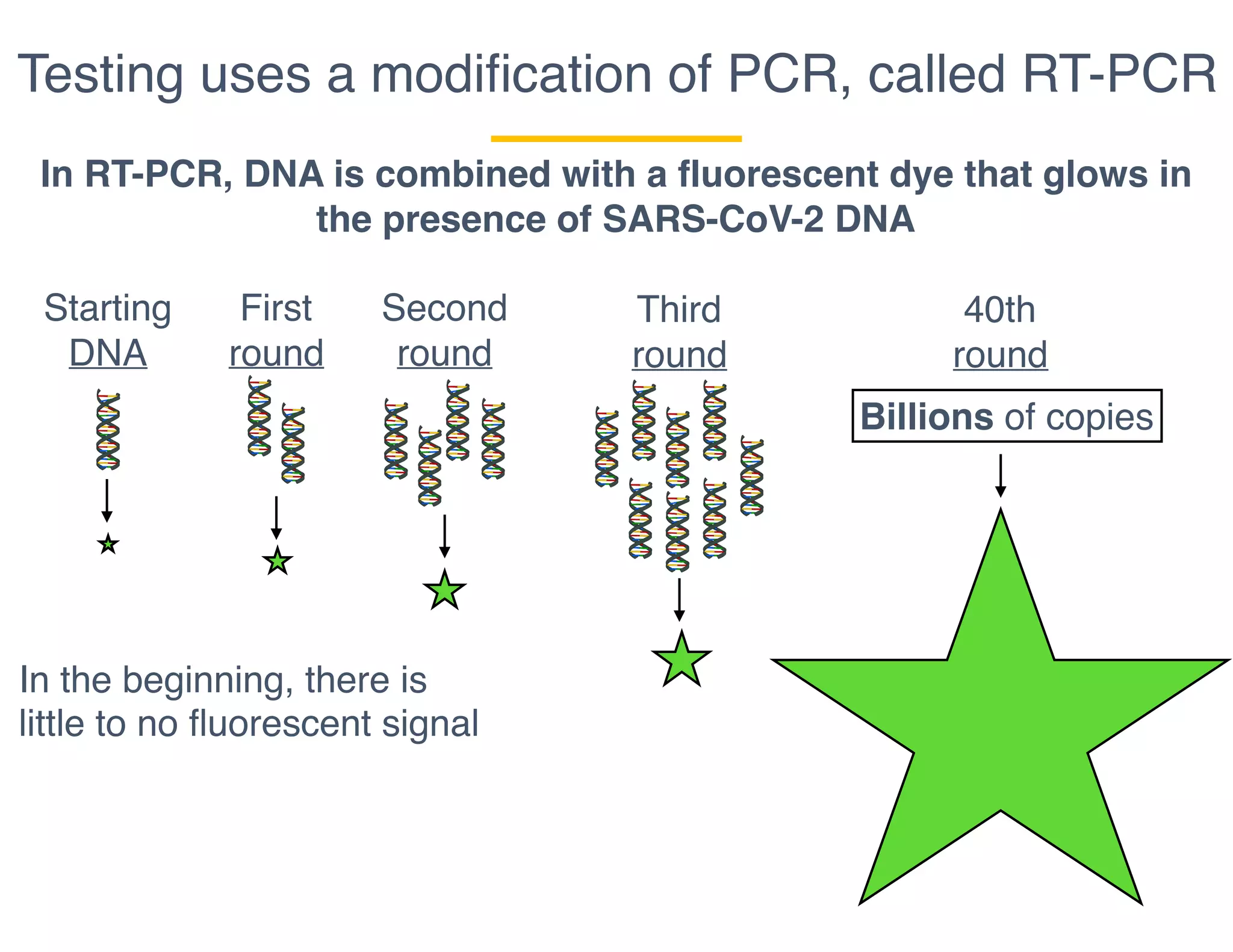
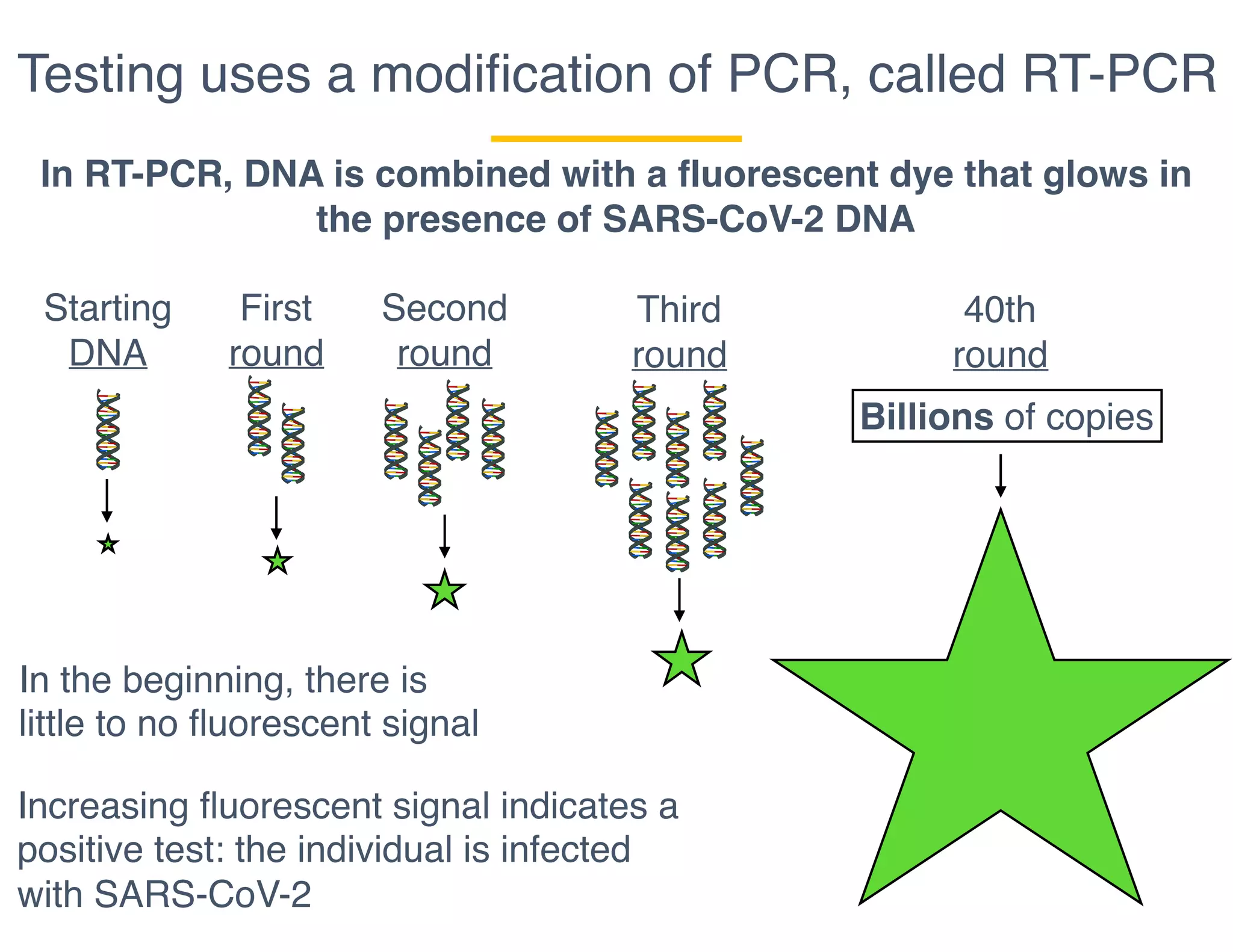
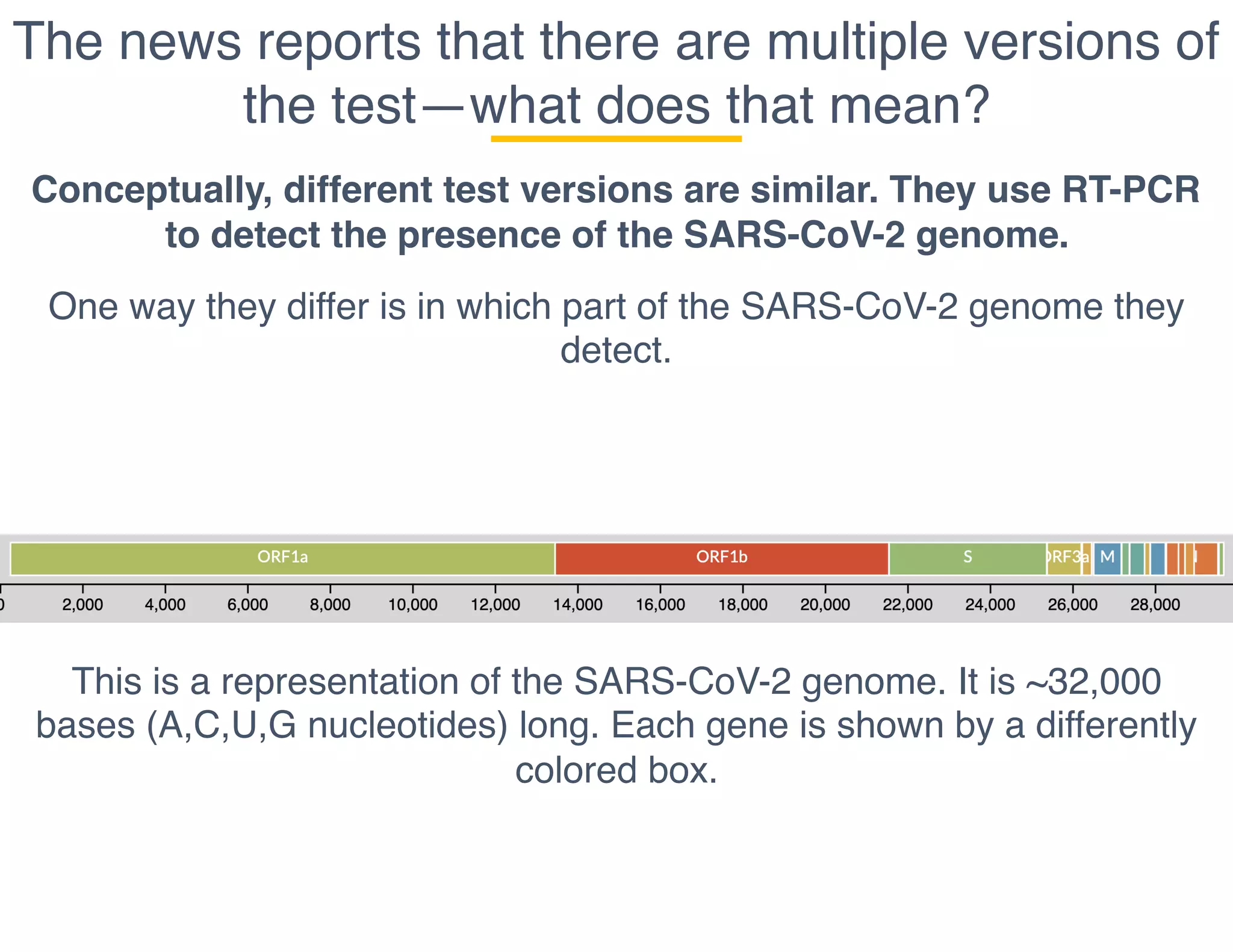
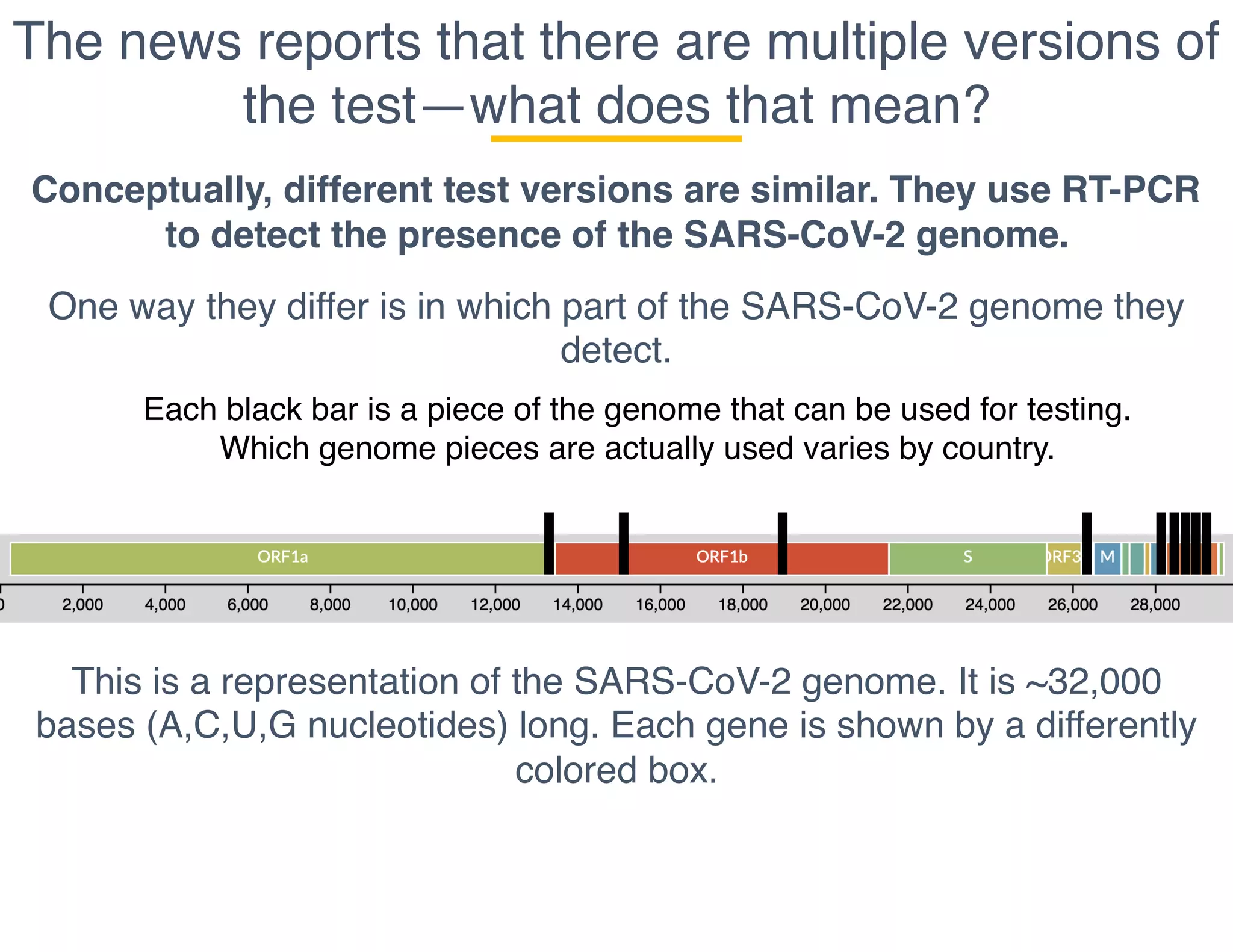
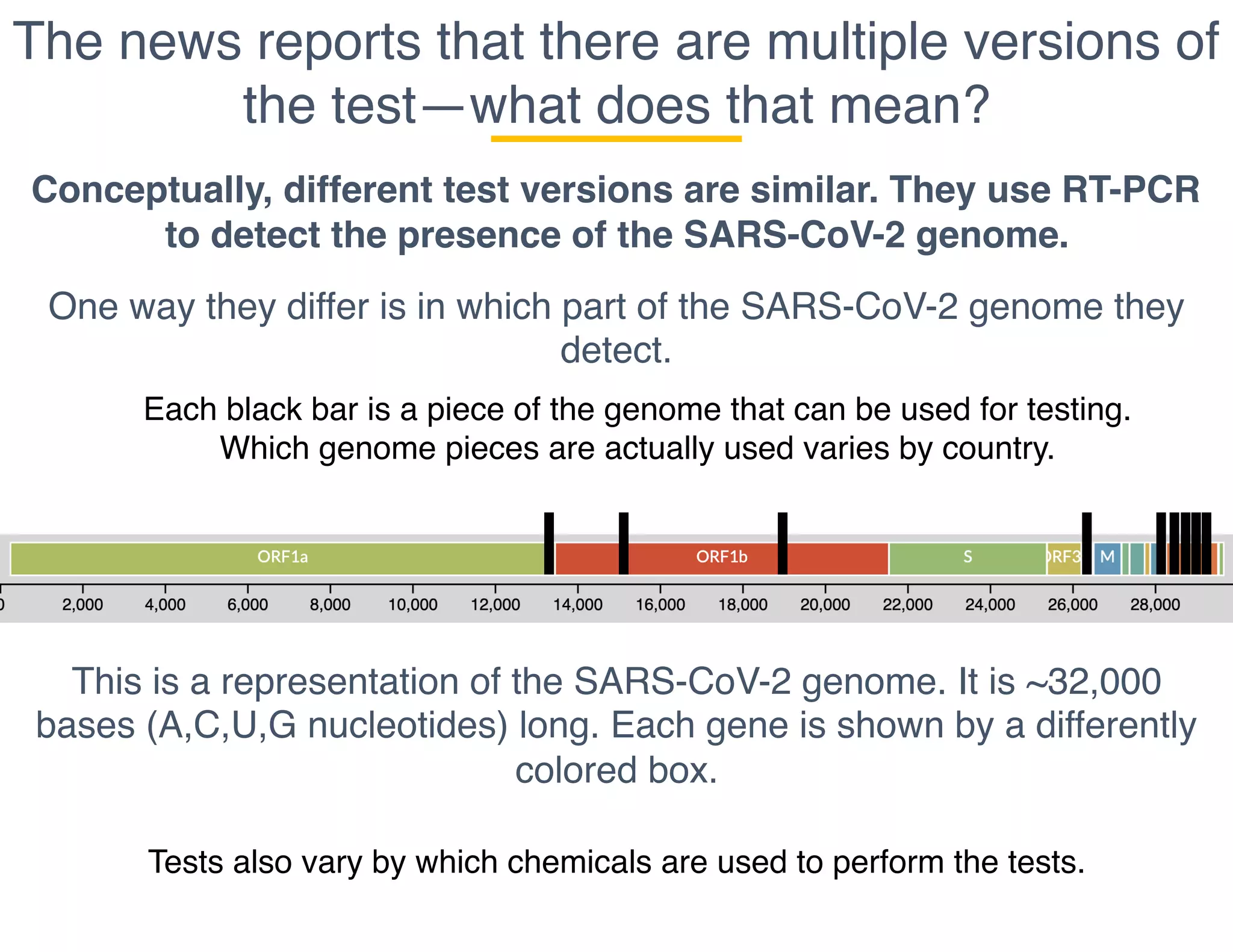
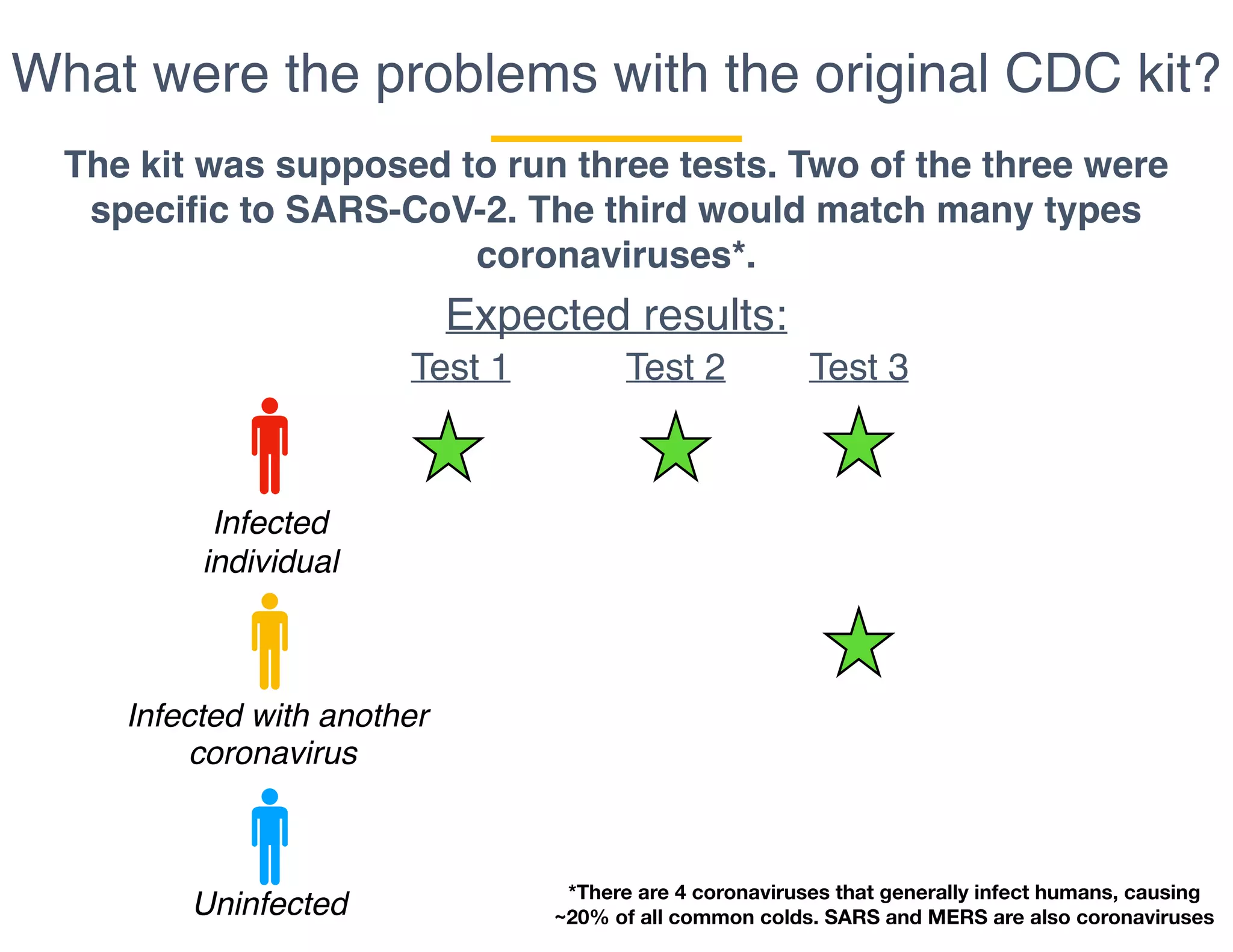
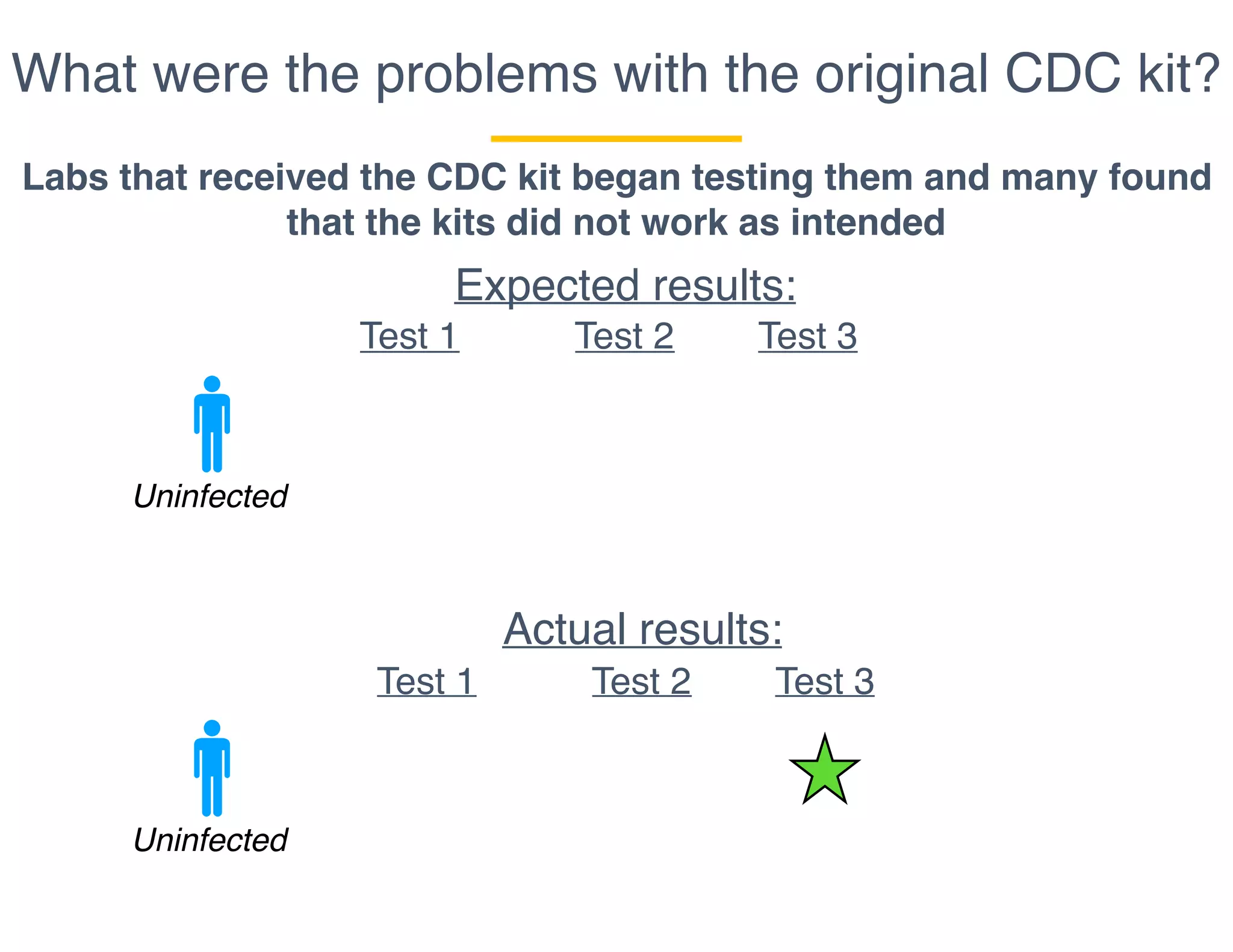
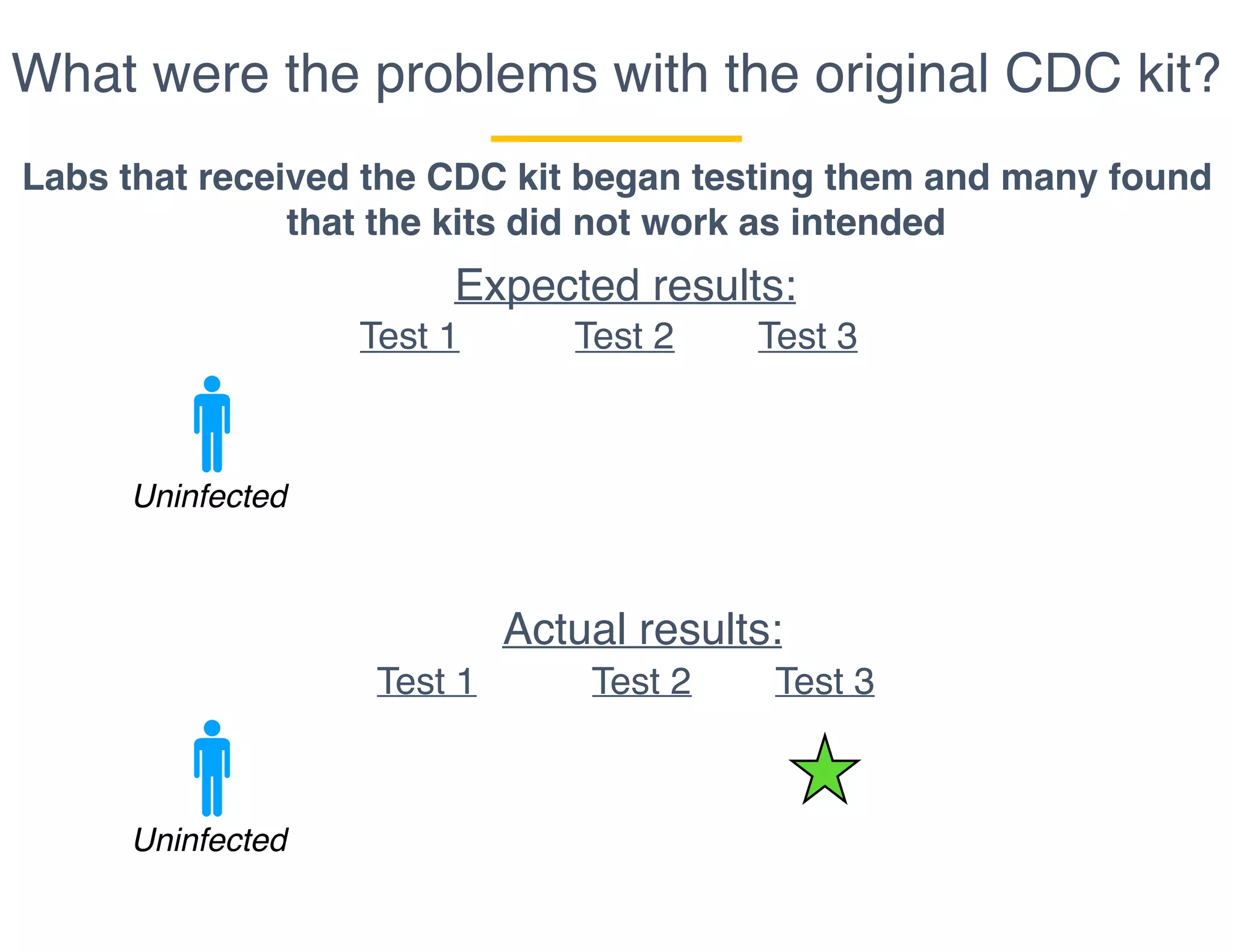
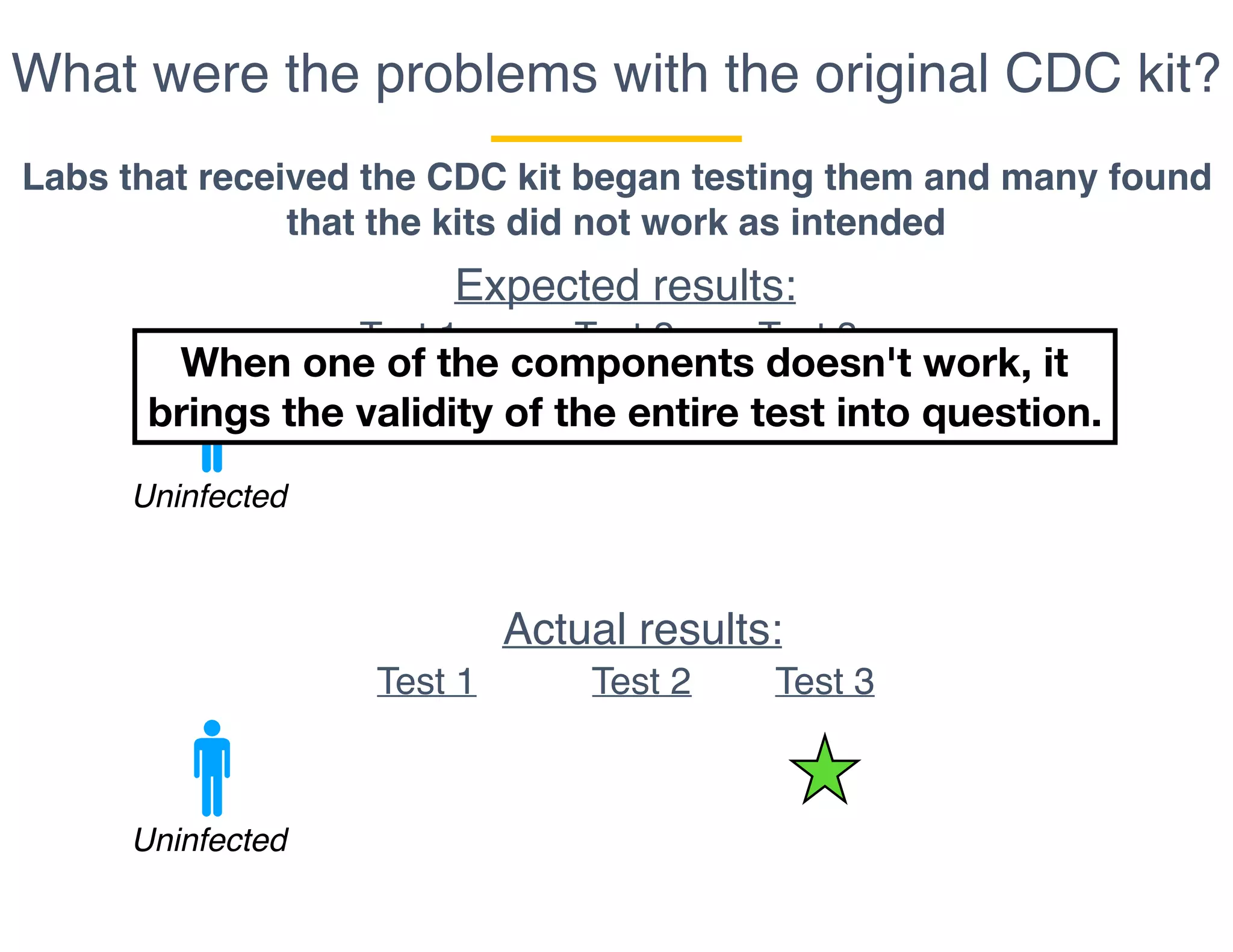
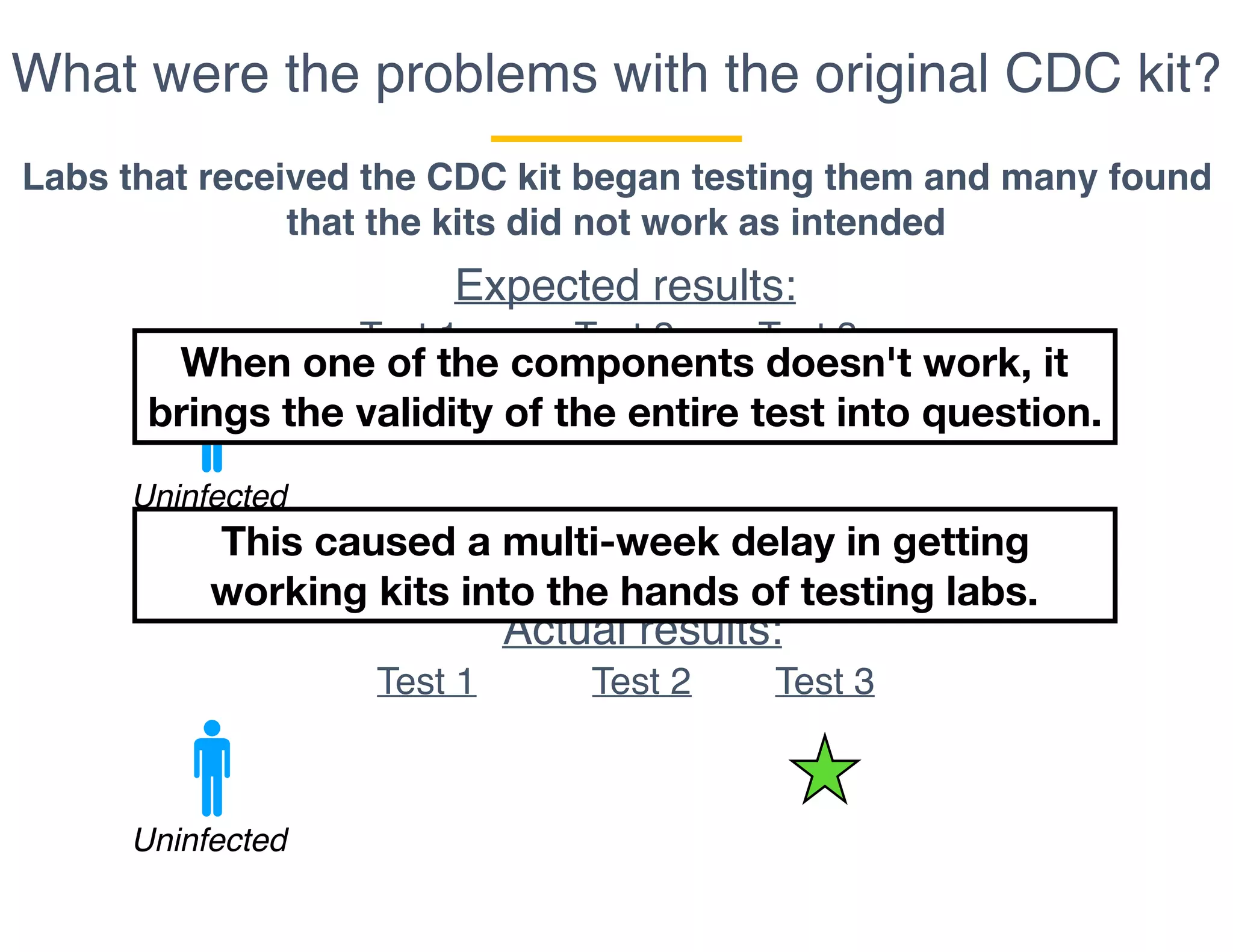
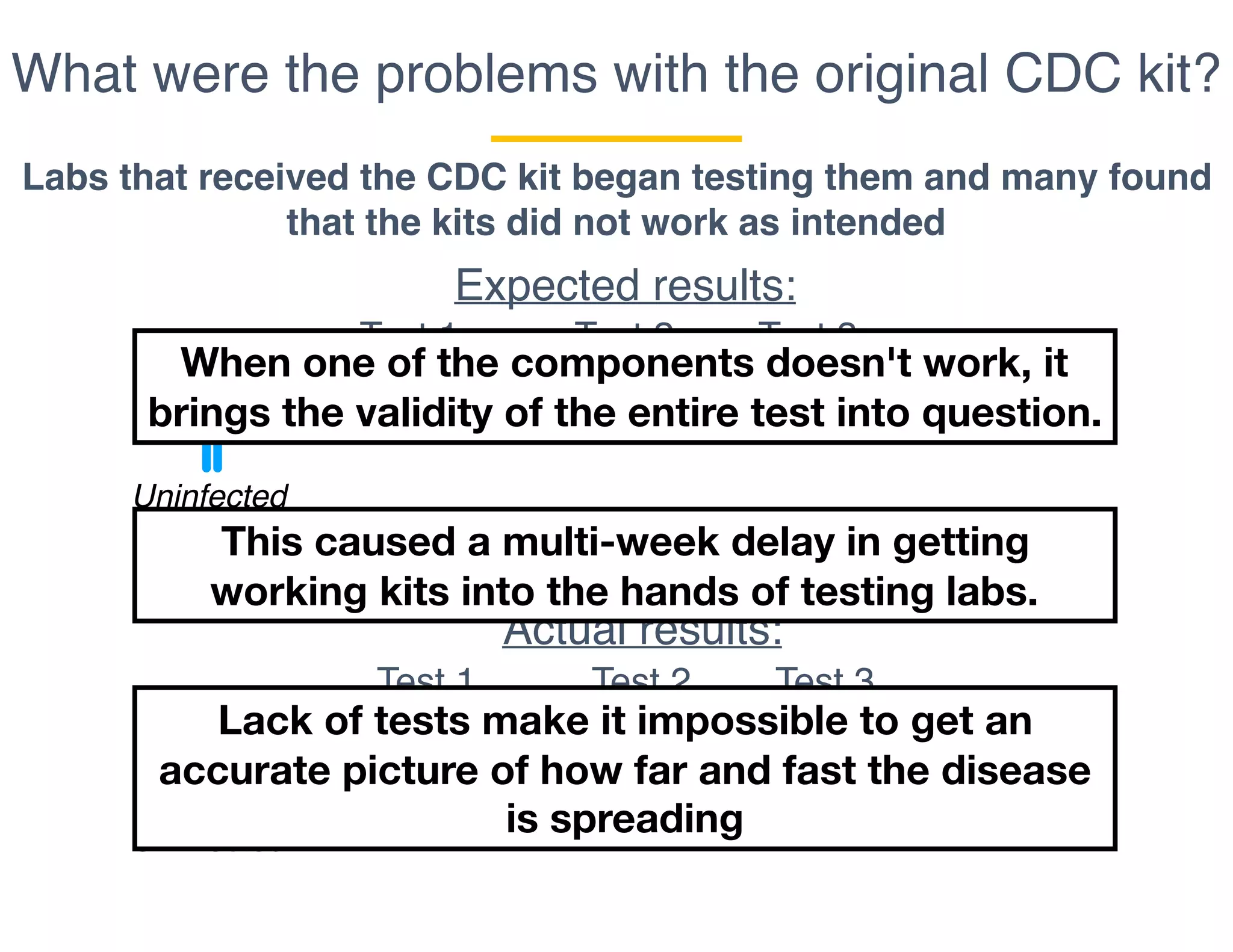
The document outlines the procedures and challenges of testing for SARS-CoV-2, emphasizing the necessity of converting RNA to DNA through reverse transcription and utilizing PCR for amplification. It discusses the various modifications of tests, shortages due to bureaucratic red tape, and issues with the original CDC test kit that hampered effective testing. Improvements in testing protocols are underway as more entities are permitted to develop and deploy their own test kits.