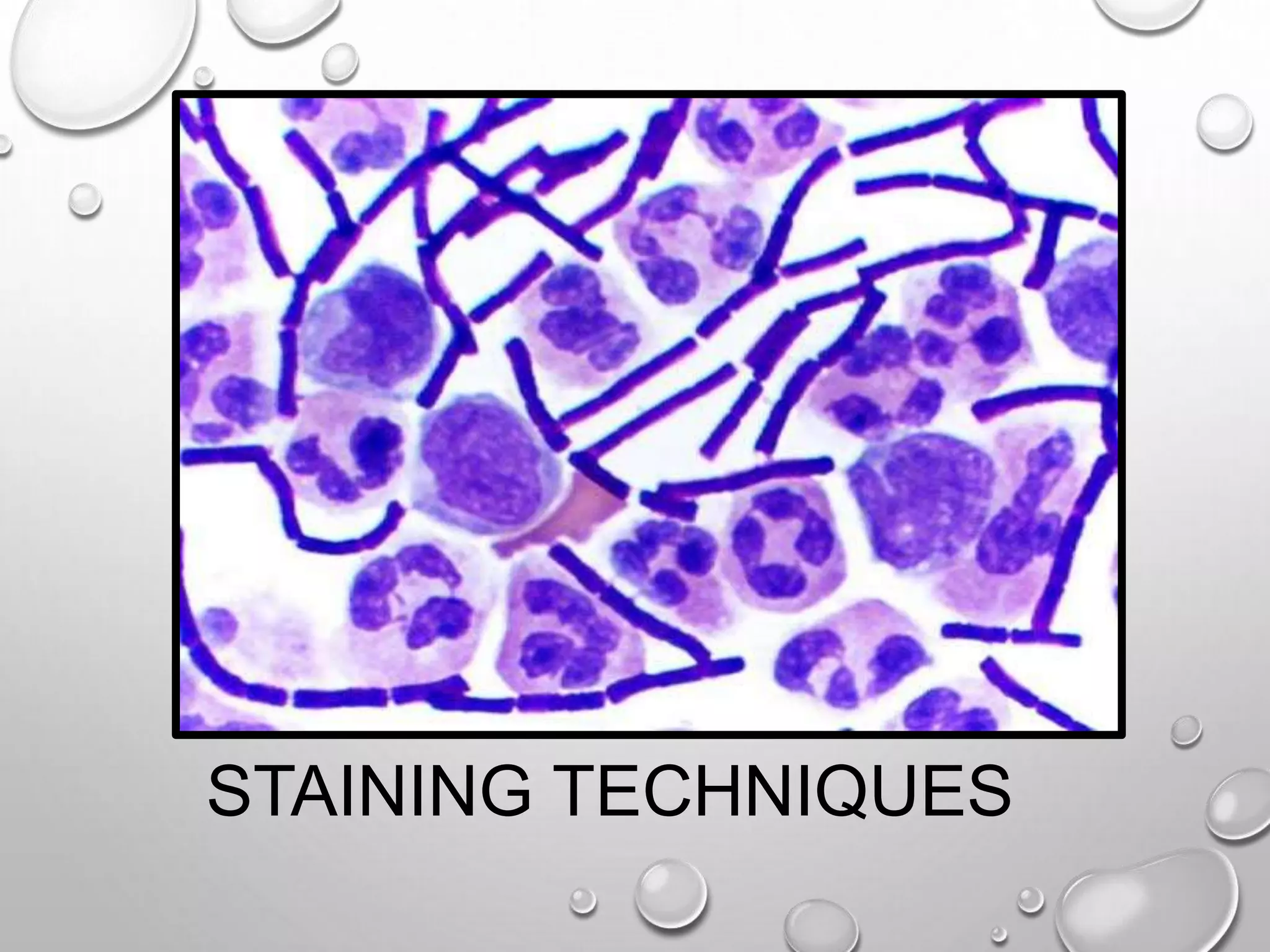
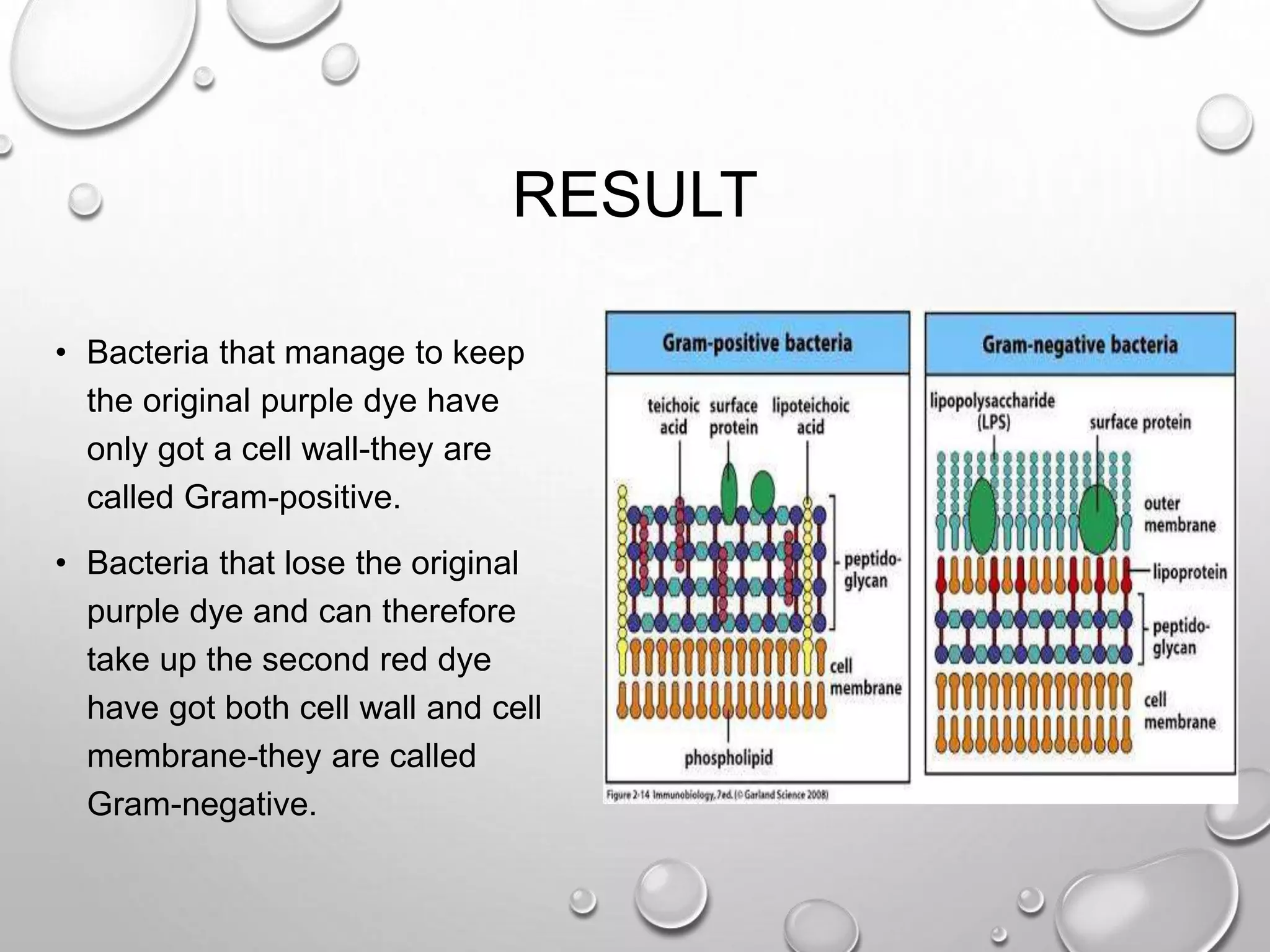
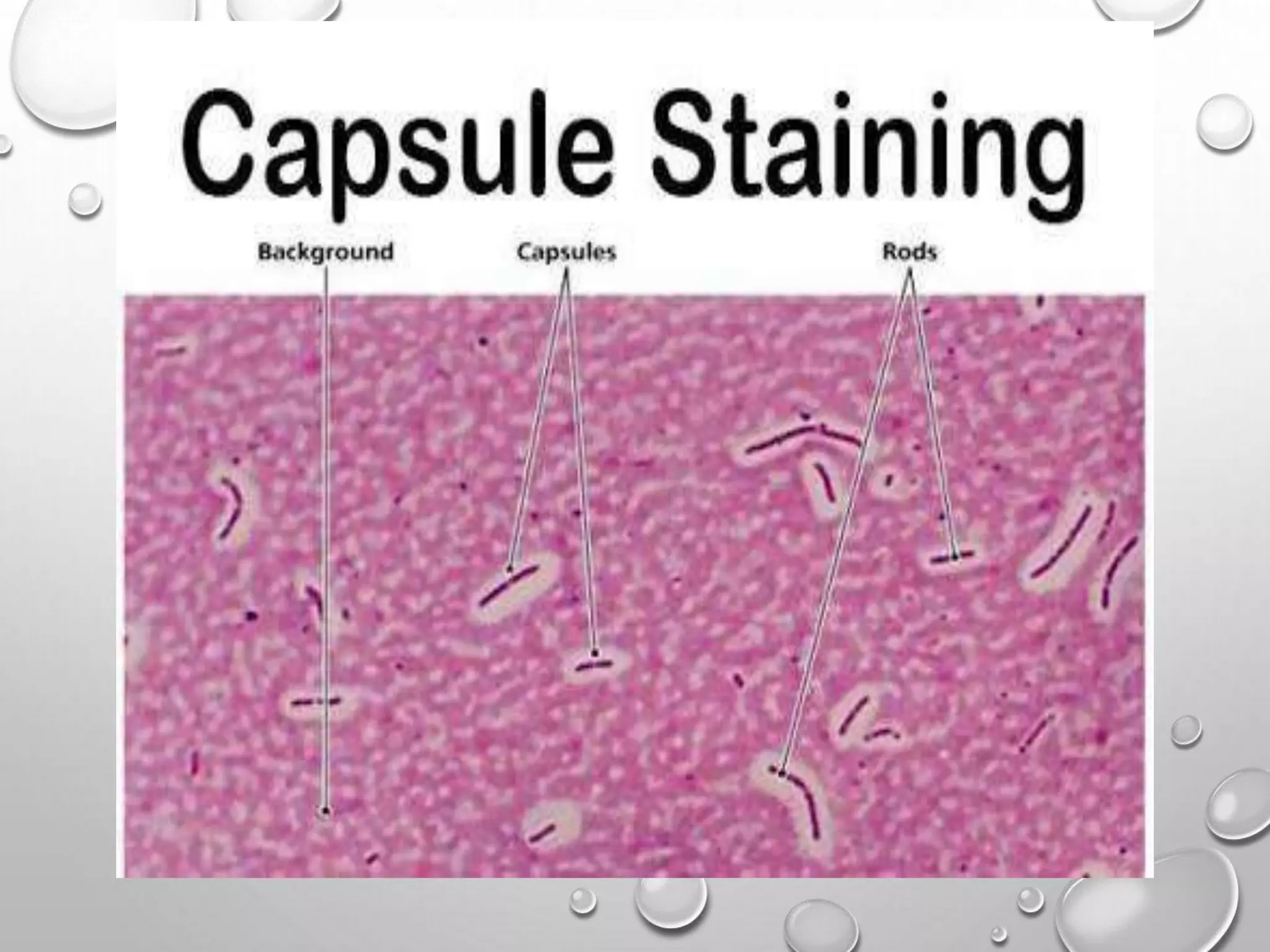
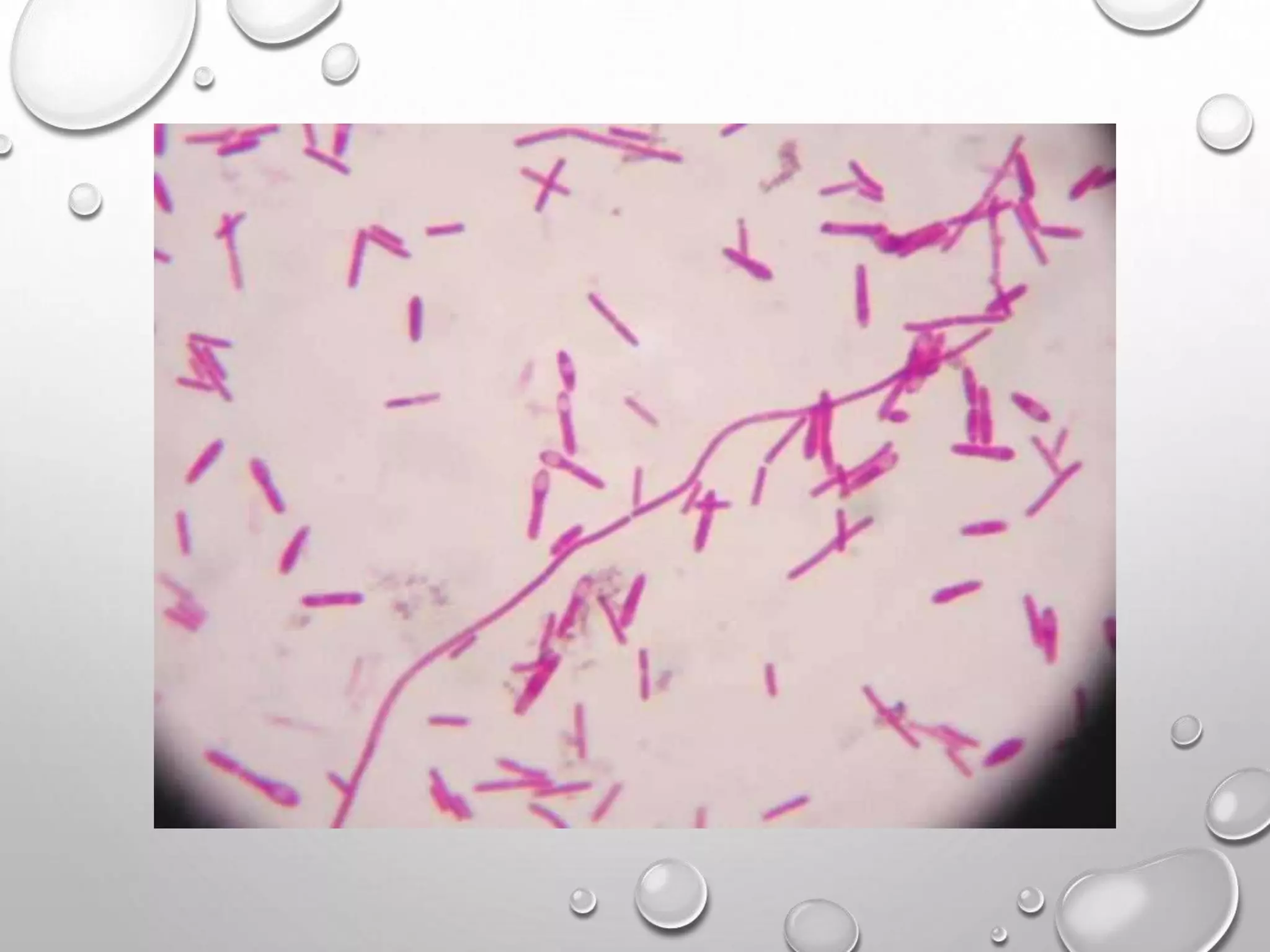
This document discusses various staining techniques used in microscopy to visualize bacteria and other microscopic organisms. It describes different types of stains including simple stains that color all structures the same and differential stains that color different structures differently. Specific staining techniques are explained, including Gram staining to distinguish between Gram-positive and Gram-negative bacteria, acid-fast staining for mycobacteria, and endospore staining. The document provides details on procedures, requirements, and results for common staining methods.