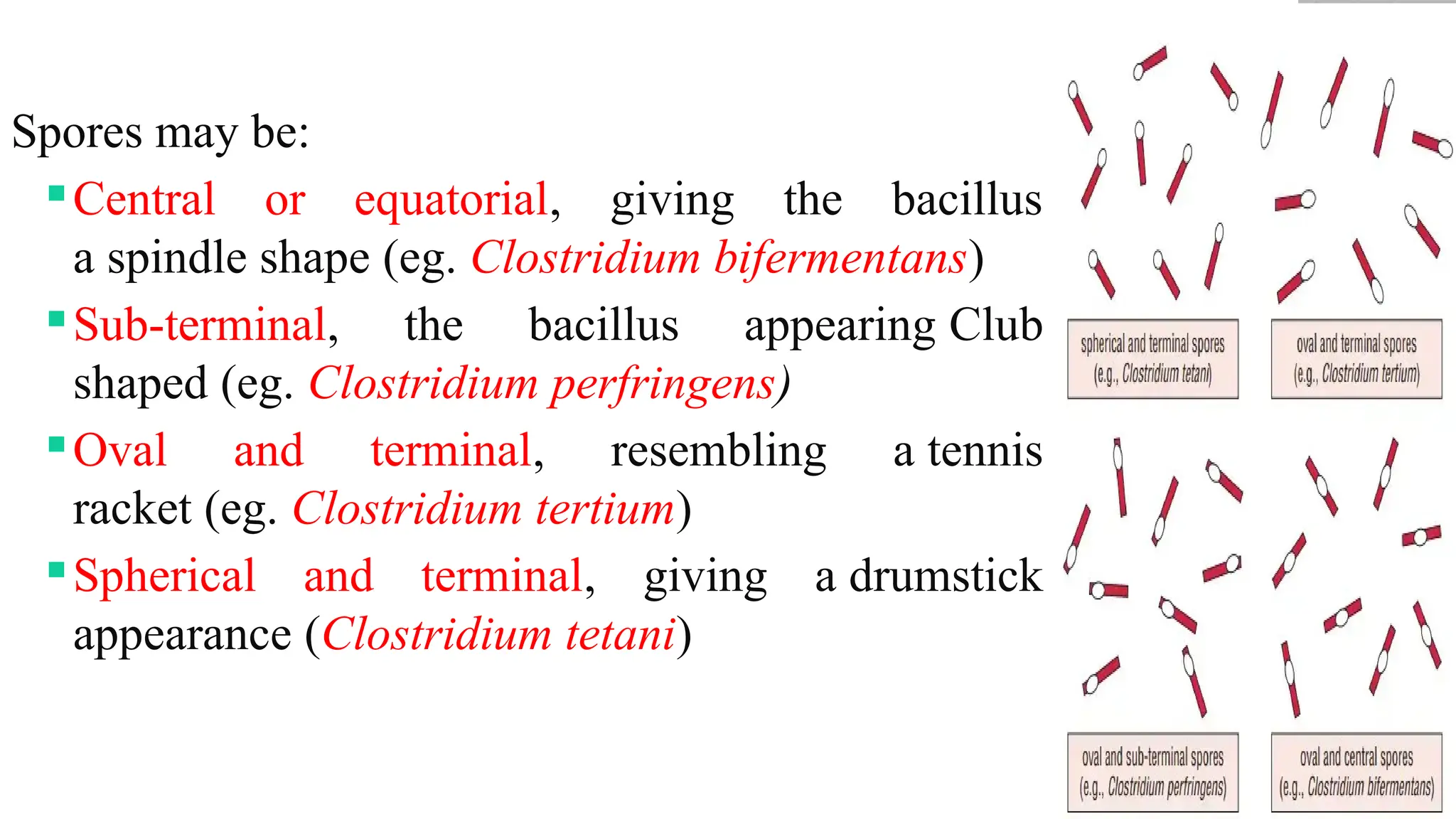
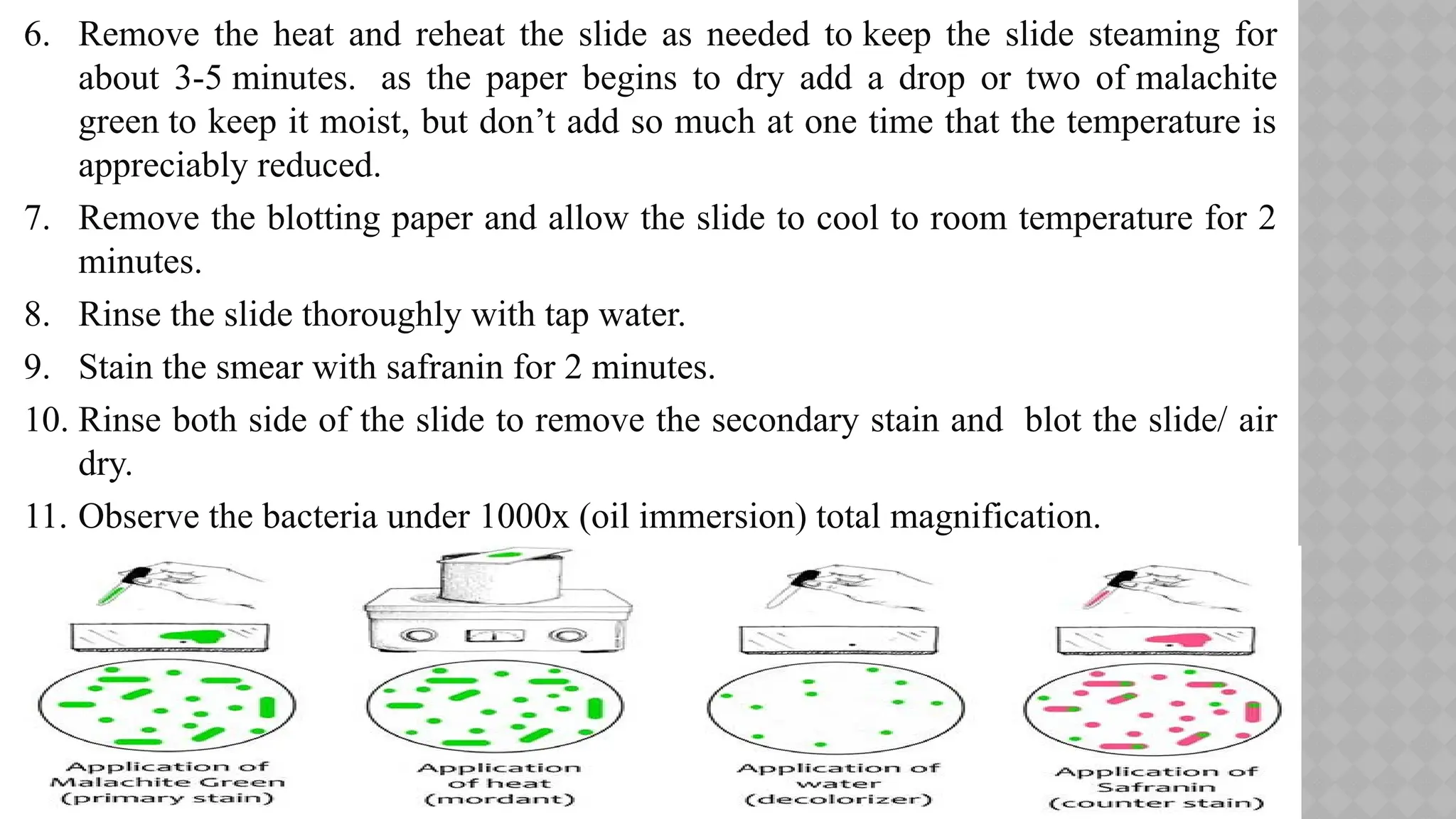
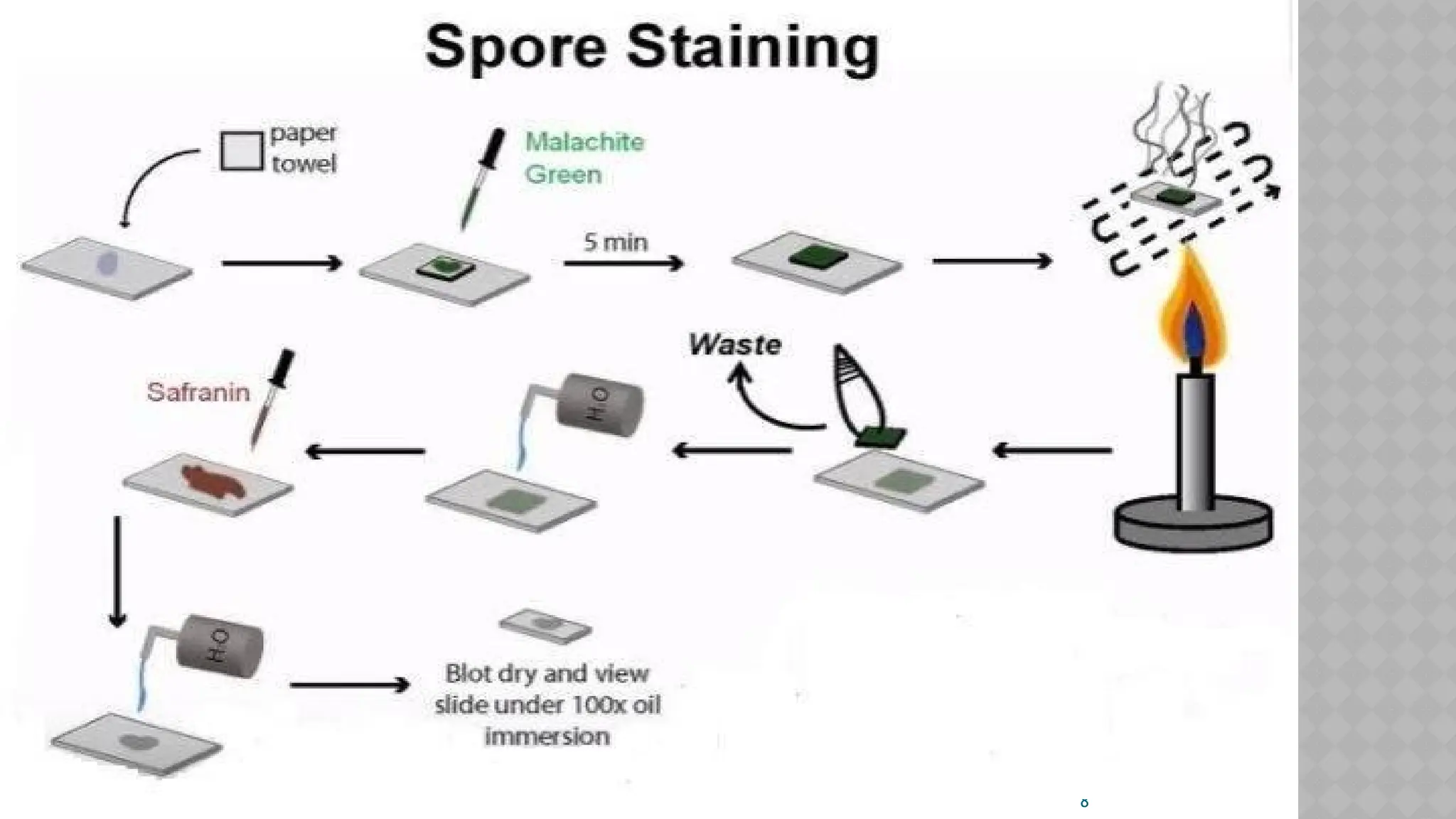
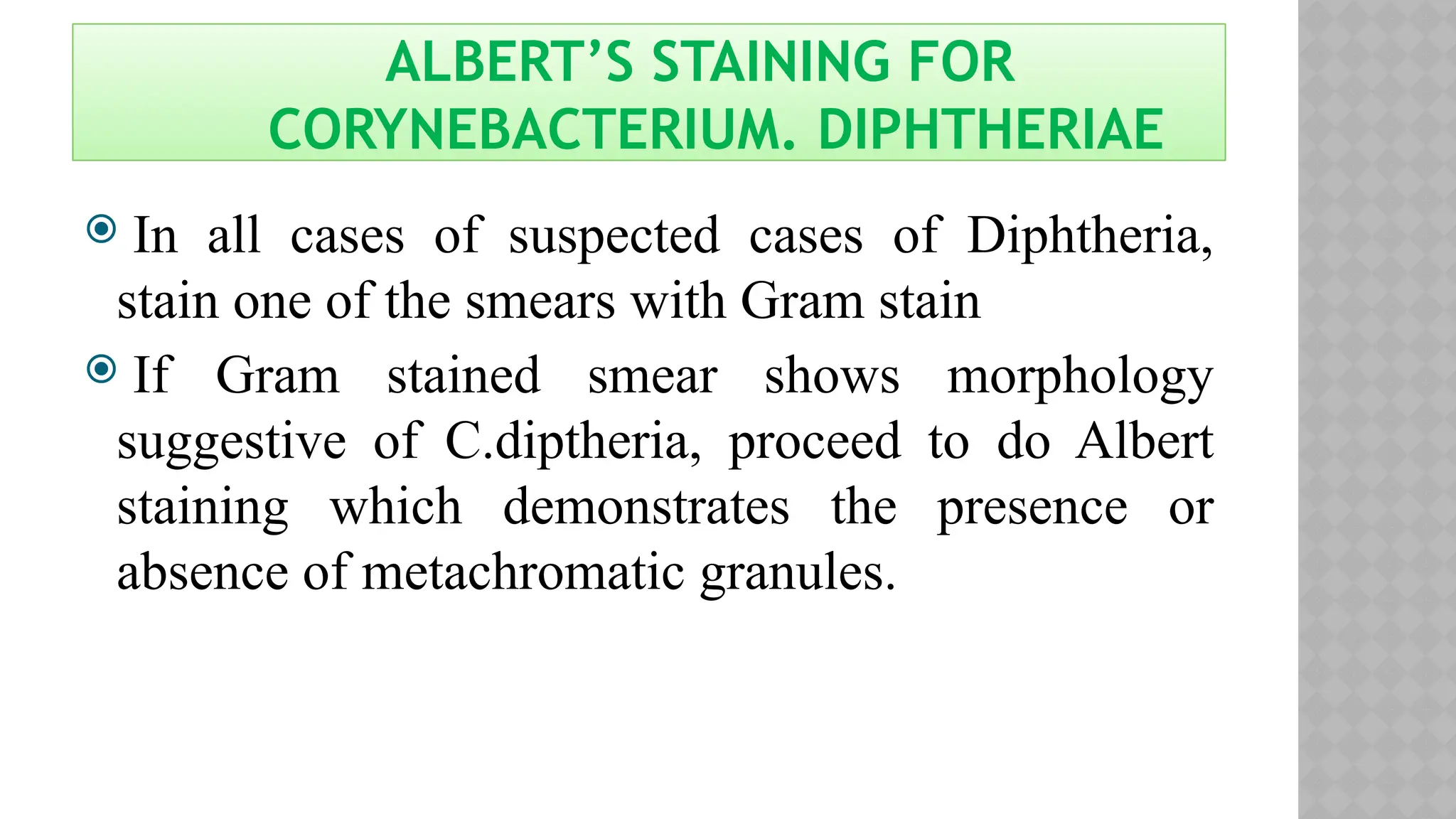
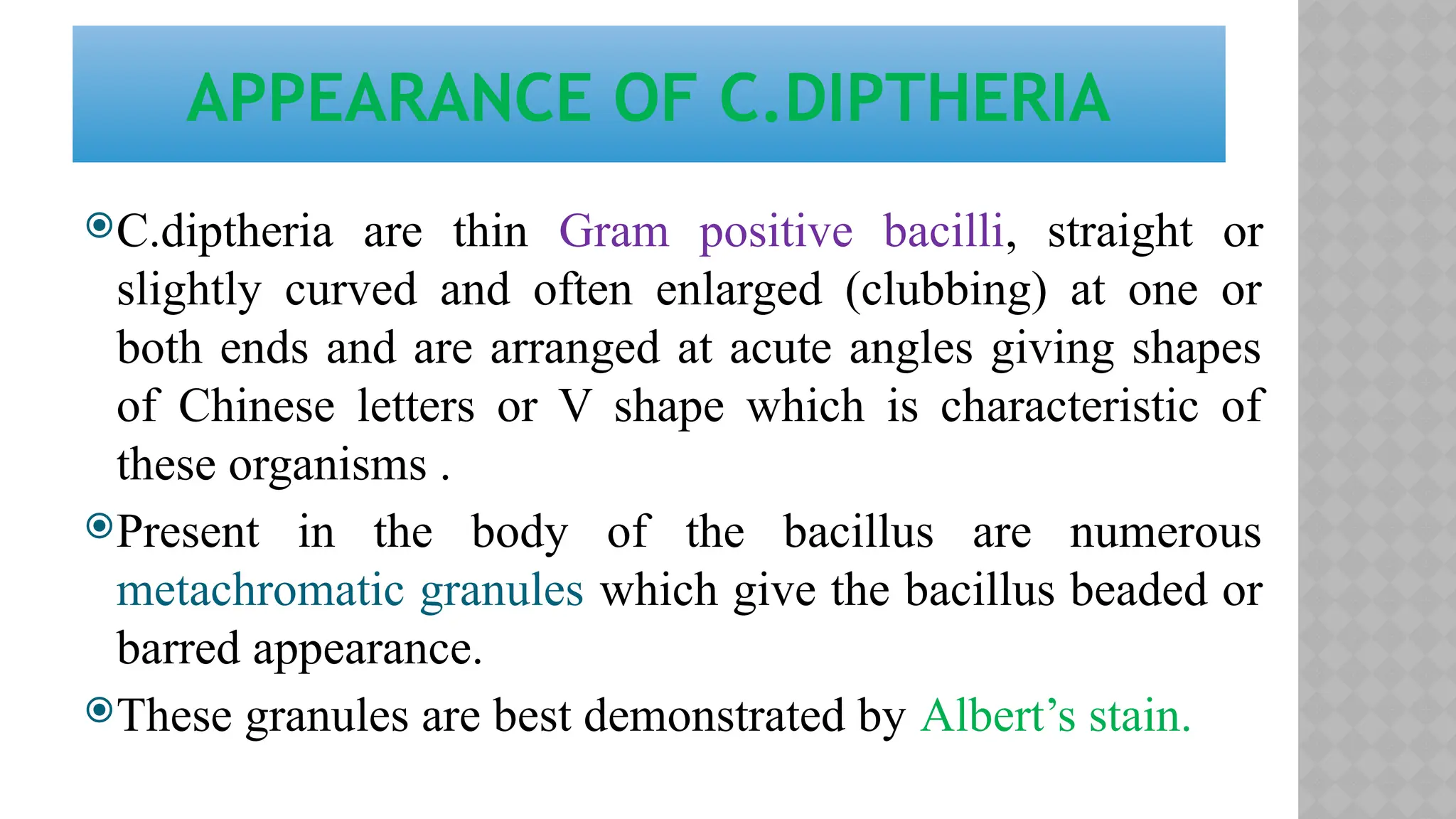
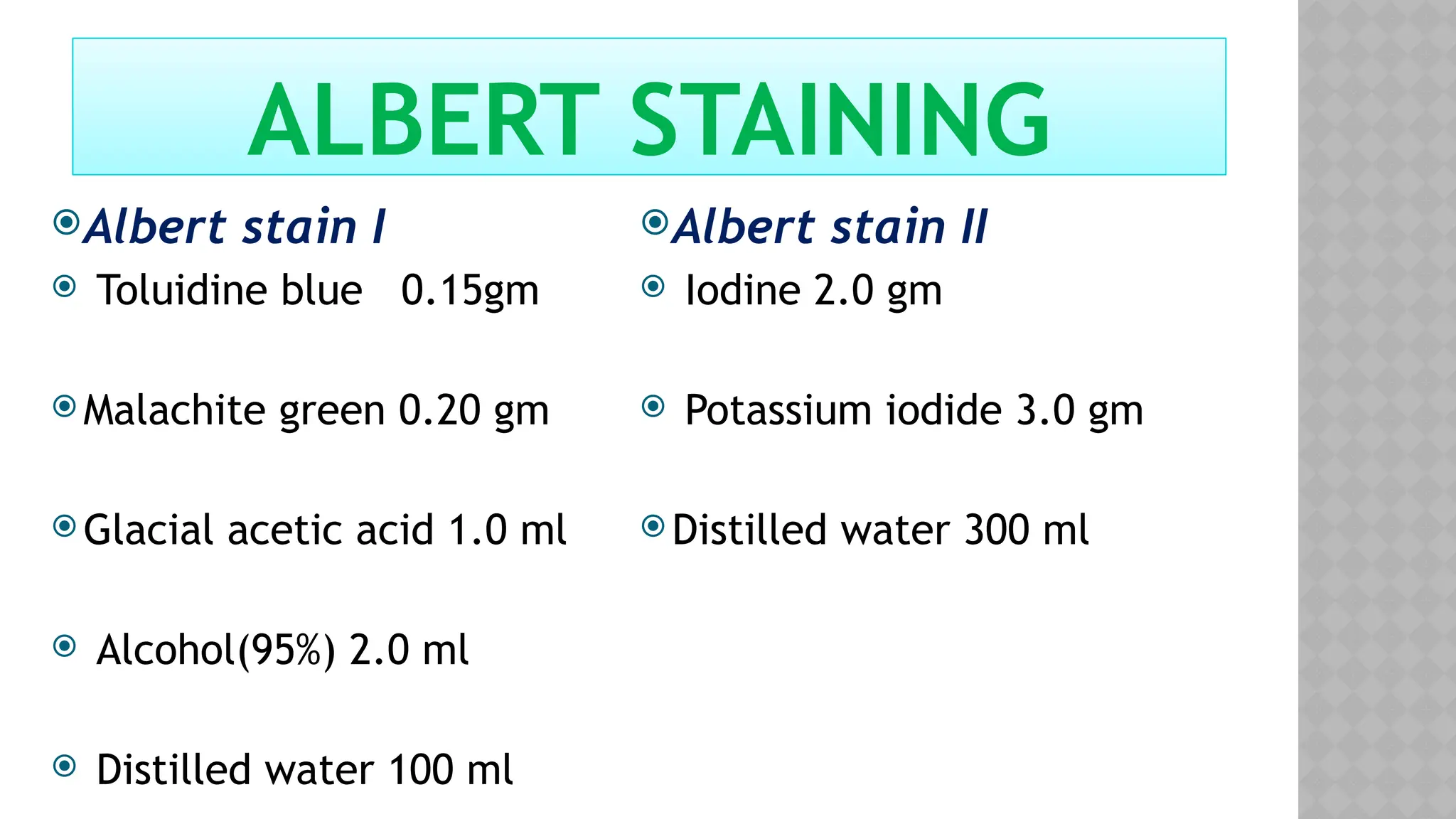
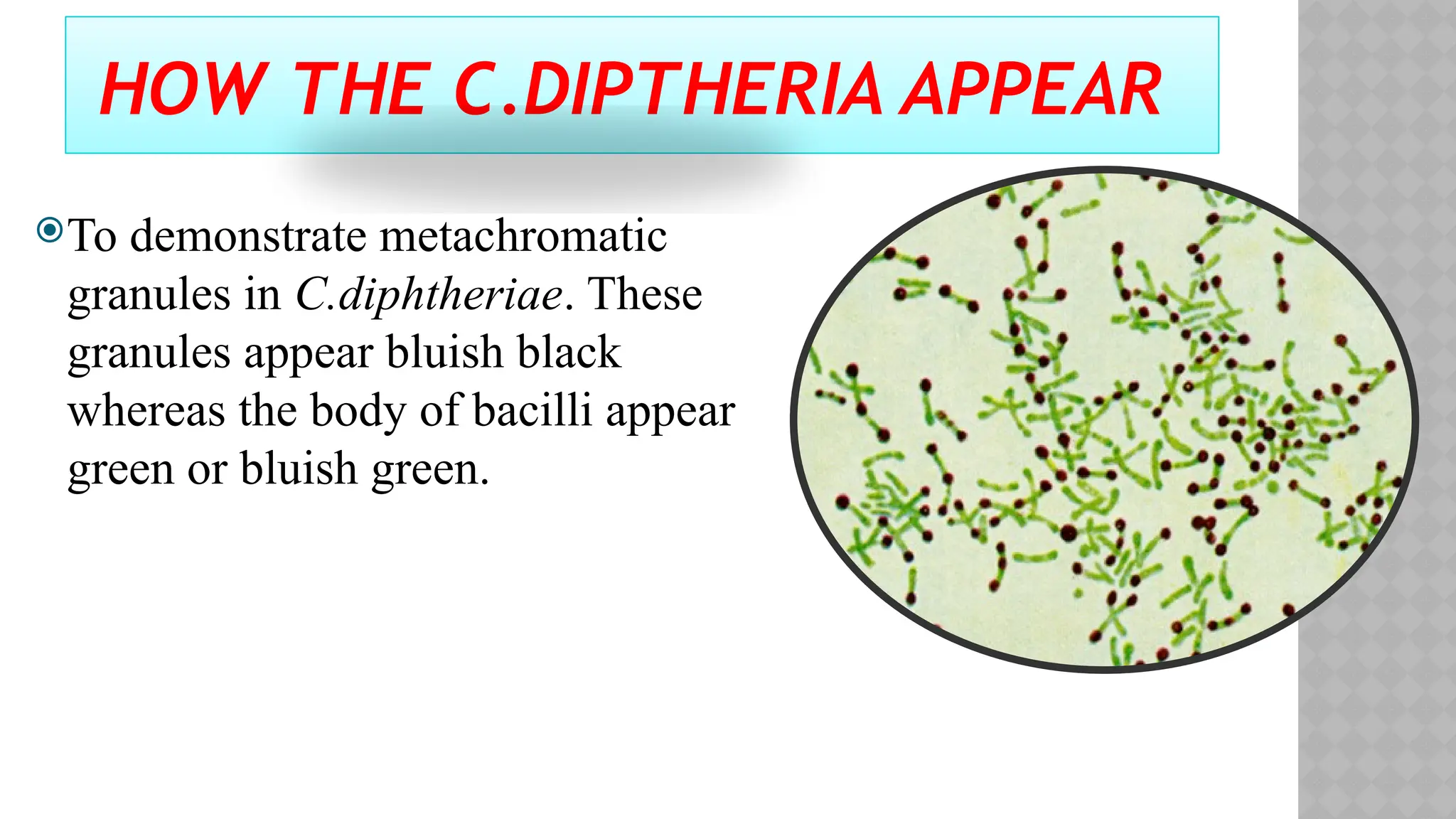
The document discusses spore staining techniques, focusing on the characteristics and classification of bacterial spores, including their heat and chemical resistance. It outlines the principles and procedures for spore staining using malachite green and safranin, as well as the detection of metachromatic granules in Corynebacterium diphtheriae using Albert's stain. The observations note the different appearances of endospores and vegetative cells post-staining, aiding in bacterial identification.